Team Leader
Hisashi Doi
D.Sci.
Laboratory for Labeling Chemistry
[Closed Mar. 2023]
E-mail hisashi.doi[at]riken.jp
Please replace [at] with @.
—Brightening Molecules—
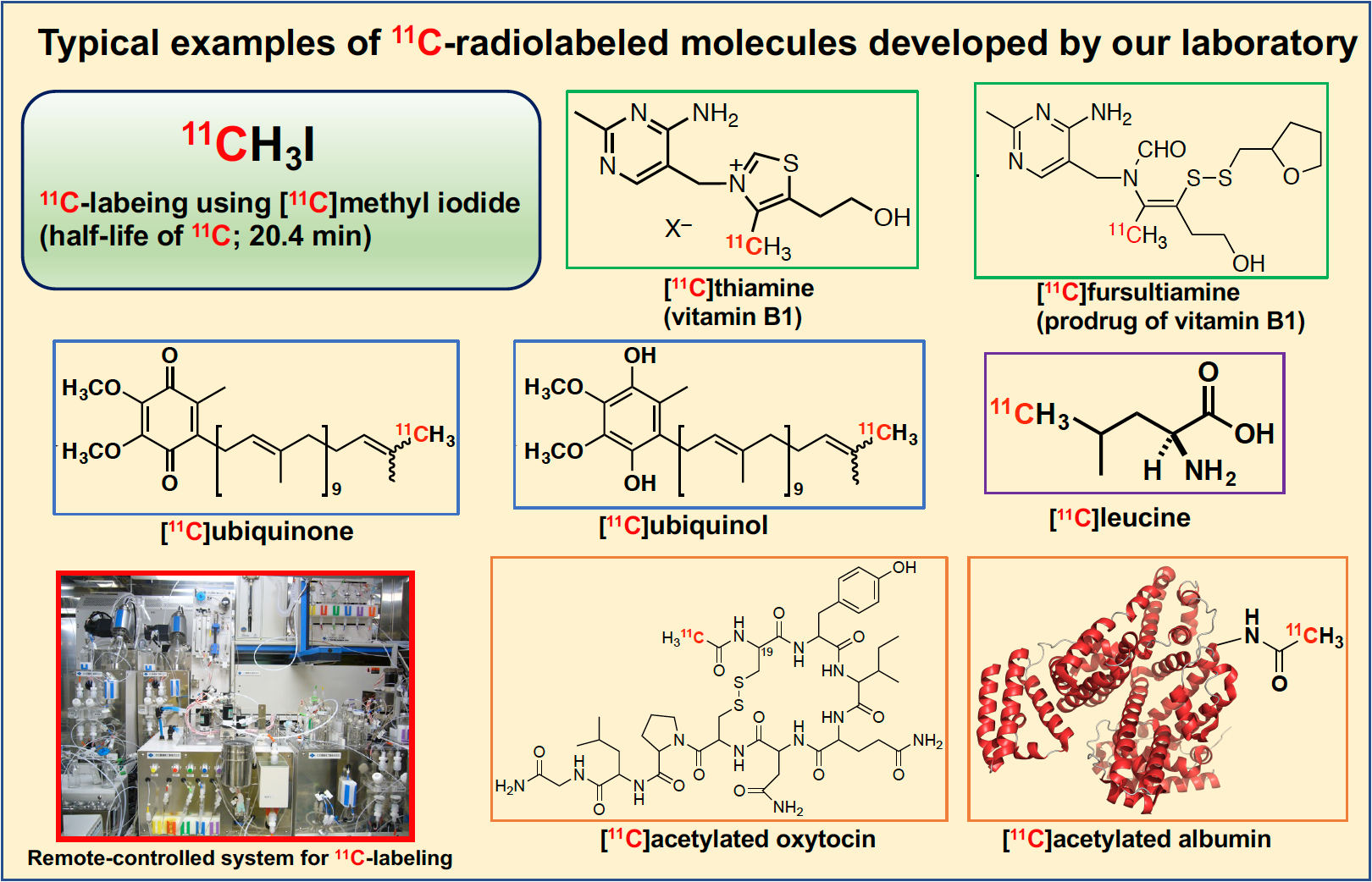
Our team is developing the general synthetic methodology of short-lived PET molecular probes for promotion of PET molecular imaging science. With the objective of applying the potentials of organic chemistry to life science, we seek to realize the new synthetic methodology of PET molecular probes by using organometallic catalysts such as Pd, Rh, and Cu etc. As one of our main projects, we are striving toward introducing11C into carbon frameworks of bioactive small organic compounds by Pd0-mediated 11C-methylationsusing [11C]CH3I. These labeling methods provides groundbreaking synthesis for introducing the [11C]methyl group into a carbon framework in the very short time of 5 minutes. We are currently developing the stoichiometry-focused18F-labeling for medium molecules such asoligodeoxynucleotides by the application of Click chemistryand 11C-acetylation for large molecules such as proteins.
In addition, our team is responsible for production of PET radiopharmaceuticals for clinical PET study in official and private hospitals.
In the field of PET radiolabeling, the performance of the remote-controlled PET probe synthesizer is of key importance for realizing high quality and high yield in the synthesis of PET molecular probes. In this regard, we are also engaged in developing original PET probe synthesizers from the perspectives of not only safety and quick operation emphasized in labeling chemistry but also introducing advantages of experimental techniques ordinarily-used in organic chemistry. Thus, we seek to realize high quality original molecular probes and establish the probe library through developing the new labeling methodology.
Research Theme
- Development of PET-probe syntheses for drug candidates and novel labeling reactions
- Development of radiolabeling system based on organic chemistry
- Development of molecular probes associated with severe diseases such as cancer, inflammation, and neurological damage
Selected Publications
Doi H, Goto M, Sato Y.
Pd0-Mediated Cross-Coupling of [11C]Methyl Iodide with Carboxysilane for Synthesis of [11C]Acetic Acid and Its Active Esters: 11C-Acetylation of Small, Medium, and Large Molecules.
European Journal of Organic Chemistry
2021(29), 3970-3979 (2021)
doi: 10.1002/ejoc.202100638
Takatani S, Tahara T, Tsuji M, et al.
Synthesis of L-[5-11 C]Leucine and L-α-[5-11 C]Methylleucine via Pd0 -mediated 11 C-Methylation and Microfluidic Hydrogenation: Potentiality of Leucine PET Probes for Tumor Imaging.
ChemMedChem
(2021)
doi: 10.1002/cmdc.202100255
Goto M, Nishiyama A, Yamaguchi T, et al.
Synthesis of 11C-labeled ubiquinone and ubiquinol via Pd0-mediated rapid C-[11C]methylation using [11C]methyl iodide and 39-demethyl-39-(pinacolboryl)ubiquinone.
Journal of Labelled Compounds and Radiopharmaceuticals
62, 86-94 (2019)
doi: 10.1002/jlcr.3700
Doi H, Kida T, Nishino K, et al.
Solubility-Improved 10-O -Substituted SN-38 derivatives with Antitumor Activity.
ChemMedChem
12, 1715-1722 (2017)
doi: 10.1002/cmdc.201700454
Doi H, Sato K, Shindou H, et al.
Blood-Brain Barrier Permeability of Ginkgolide: Comparison of the Behavior of PET Probes 7α-{18 F]Fluoro- and 10-O -p -[11 C]Methylbenzyl Ginkgolide B in Monkey and Rat Brains.
Bioorganic & Medicinal Chemistry
24, 5148-5157 (2016)
doi: 10.1016/j.bmc.2016.08.032
Doi H, Mawatari A, Kanazawa M, et al.
Synthesis of 11 C-Labeled Thiamine and Fursultiamine for in Vivo Molecular Imaging of Vitamin B1 and Its Prodrug Using Positron Emission Tomography.
The Journal of Organic Chemistry
80(12), 6250-6258 (2015)
doi: 10.1021/acs.joc.5b00685
Doi H.
Pd-mediated rapid cross-couplings using [11 C]methyl iodide: groundbreaking labeling methods in 11 C radiochemistry.
Journal of Labelled Compounds and Radiopharmaceuticals
58(3), 73-85 (2015)
doi: 10.1002/jlcr.3253
Kuboyama T, Nakahara M, Yoshino M, et al.
Stoichiometry-focused 18F-labeling of alkyne-substituted oligodeoxynucleotides using azido([18 F]fluoromethyl)benzenes by Cu-catalyzed Huisgen reaction.
Bioorganic & Medicinal Chemistry
19(1), 249-255 (2011)
doi: 10.1016/j.bmc.2010.11.033
Doi H, Ban I, Nonoyama A, et al.
Palladium(0)-mediated rapid methylation and fluoromethylation on carbon frameworks by reacting methyl and fluoromethyl iodide with aryl and alkenyl boronic acid esters: useful for the synthesis of [11 C]CH3 --C- and [18 F]FCH2 --C-Containing PET tracers (PET=positron emission tomography).
Chemistry
15(16), 4165-4171 (2009)
doi: 10.1002/chem.200801974



