
Project Leader
(Apr. 2018—Jul. 2019)
Masayo Takahashi
M.D., Ph.D.
(Aug. 2019—Mar. 2022)
Eisuke Nishida
Ph.D.
Laboratory for Retinal Regeneration
[Closed Mar. 2022]
The retina has been called the "approachable part of the brain," owing to its relatively simple structure and its location near the body surface, and for these reasons it serves as a useful and experimentally amenable model of the central nervous system. Until very recently, it was thought that in adult mammals the retina was entirely incapable of regenerating, but we now know that at least new retinal neurons can be generated after being damaged. This has opened up new hope that the adult retina may retain the ability to regenerate neurons and even to reconstitute the neural network. We are now exploring the exciting prospect that, by transplanting cells from outside of the retina or by regeneration from intrinsic progenitor cells, it may one day be possible to restore lost function to damaged retinas.
Our research into retinal regeneration seeks to achieve clinical applications by developing methods for inducing stem cells or embryonic stem cells to differentiate into retinal neurons and pigment epithelial cells in sufficient quantities for use in the treatment of patients suffering from conditions in which such cells have been damaged or lost. We must also ensure that such cells establish viable grafts upon transplantation and induce the reconstitution of functional neural networks. We also hope to develop means of promoting true regeneration by activating endogenous stem cells to replace cells lost to trauma or disease and thus repair damaged tissues. Access to a broad spectrum of developmental biological research information will be key to the achievement of these goals, and we appreciate the opportunities for exchange that working in the environment provided by the RIKEN BDR.
Therapeutic applications cannot be developed from basic research alone; the clinical approach - a thorough understanding of the medical condition to be treated is equally important. For conditions such as retinitis pigmentosa, even the successful transplantation of cells in animal models may not necessarily be translatable to a human clinical therapy without an understanding of the underlying genetics and possible immunological involvement. Our goal is to study retinal regeneration based on both a strong foundation in basic research and solid clinical evidence.
Research Theme
- Retinal cell transplantation
- Genetic diagnosis of retinitis pigmentosa
- Relationship between photoreceptor death and environment in retinitis pigmentosa
- Development of regenerative medicine system
- iPSC-derived retinal ganglion cell
Selected Publications
Kanda GN, Tsuzuki T, Terada M, et al.
Robotic search for optimal cell culture in regenerative medicine.
eLife
11, e77007 (2022)
doi: 10.7554/eLife.77007
Yamasaki S, Tu HY, Matsuyama T, et al.
A Genetic modification that reduces ON-bipolar cells in hESC-derived retinas enhances functional integration after transplantation.
iScience
25(1), 103657 (2022)
doi: 10.1016/j.isci.2021.103657
Ochiai K, Motozawa N, Terada M, et al.
A Variable Scheduling Maintenance Culture Platform for Mammalian Cells.
SLAS technology
26(2), 209-217 (2021)
doi: 10.1177/2472630320972109
Takahashi TM, Sunagawa GA, Soya S, et al.
A discrete neuronal circuit induces a hibernation-like state in rodents.
Nature
583, 109-114 (2020)
doi: 10.1038/s41586-020-2163-6
Matsumoto E, Koide N, Hanzawa H, et al.
Fabricating retinal pigment epithelial cell sheets derived from human induced pluripotent stem cells in an automated closed culture system for regenerative medicine.
PloS one
14(3), e0212369 (2019)
doi: 10.1371/journal.pone.0212369
Kime C, Kiyonari H, Ohtsuka S, et al.
Induced 2C Expression and Implantation-Competent Blastocyst-like Cysts from Primed Pluripotent Stem Cells.
Stem Cell Reports
13(3), 485-498 (2019)
doi: 10.1016/j.stemcr.2019.07.011
Kitahata S, Tanaka Y, Hori K, et al.
Critical Functionality Effects from Storage Temperature on Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium Cell Suspensions.
Scientific Reports
9, 2891 (2019)
doi: 10.1038/s41598-018-38065-6
Tu HY, Watanabe T, Shirai H, et al.
Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration.
EBioMedicine
39, 562-574 (2019)
doi: 10.1016/j.ebiom.2018.11.028
Goto S, Onishi A, Misaki K, et al.
Neural retina-specific Aldh1a1 controls dorsal choroidal vascular development via Sox9 expression in retinal pigment epithelial cells.
eLife
7, e32358 (2018)
doi: 10.7554/eLife.32358.001
Iraha S, Tu HY, Yamasaki S, et al.
Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation.
Stem Cell Reports
10, 1059-1074 (2018)
doi: 10.1016/j.stemcr.2018.01.032
Mandai M, Watanabe A, Kurimoto Y, et al.
Autologous induced stem-cell-derived retinal cells for macular degeneration.
The New England Journal of Medicine
376, 1038-1046 (2017)
doi: 10.1056/NEJMoa1608368
Mandai M, Fujii M, Hashiguchi T, et al.
iPSC-derived retinal transplants improve vision in rd1 end-stage retinal degeneration mice.
Stem Cell Reports
8, 69-83 (2017)
doi: 10.1016/j.stemcr.2016.12.008
Sugita S, Iwasaki Y, Makabe K, et al.
Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models.
Stem Cell Reports
7(4), 635-648. (2016)
doi: 10.1016/j.stemcr.2016.08.010
Shirai H, Mandai M, Matsushita K, et al.
Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration.
Proceedings of the National Academy of Sciences of the United States of America
113(1), E81-90 (2016)
doi: 10.1073/pnas.1512590113
Kamao H, Mandai M, Okamoto S, et al.
Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application.
Stem Cell Reports
2, 205-218 (2014)
doi: 10.1016/j.stemcr.2013.12.007
Jin ZB, Okamoto S, Osakada F, et al.
Modeling retinal degeneration using patient-specific induced pluripotent stem cells.
PLoS One
6, e17084 (2011)
doi: 10.1371/journal.pone.0017084
News
Jan. 27, 2022 Research
Improved retinal transplant technique almost ready for clinical trials

Jul. 9, 2021 BDR News
Masayo Takahashi awarded rank of Chevalier in France’s National Order of Merit

Jun. 15, 2021 BDR News
Senior visiting scientist Masayo Takahashi elected as EMBO Associate Member

Jan. 8, 2021 BDR News
RIKEN People
Helping regenerative ideas flourish

Dec. 25, 2020 Research
Hijacking hibernation

Jun. 12, 2020 Research
Hibernation in mice: Are humans next?

Apr. 1, 2020 BDR News
Dive into BDR's intriguing research
A new scheme of science

Jan. 30, 2020 BDR News
Public Events
Booth at local science fair
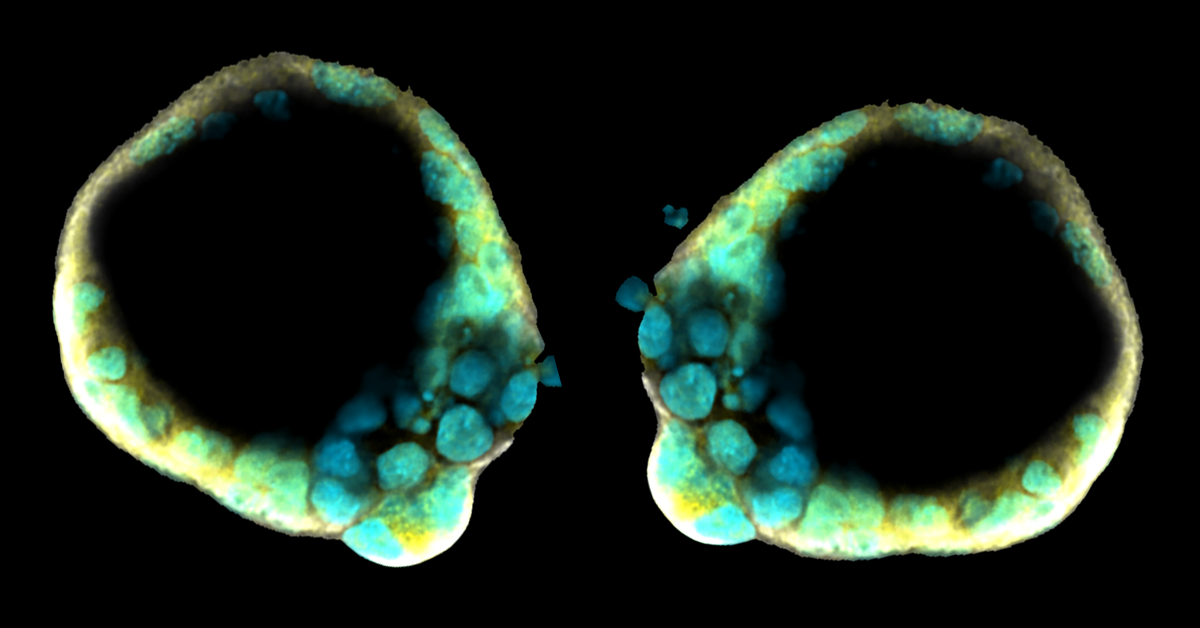
Aug. 9, 2019 Research
Implantable 3D blastocyst-like embryonic structure generated from mouse stem cells

Jun. 28, 2019 BDR News
RIKEN People
Leading the race to regenerate eyes



