General research interests
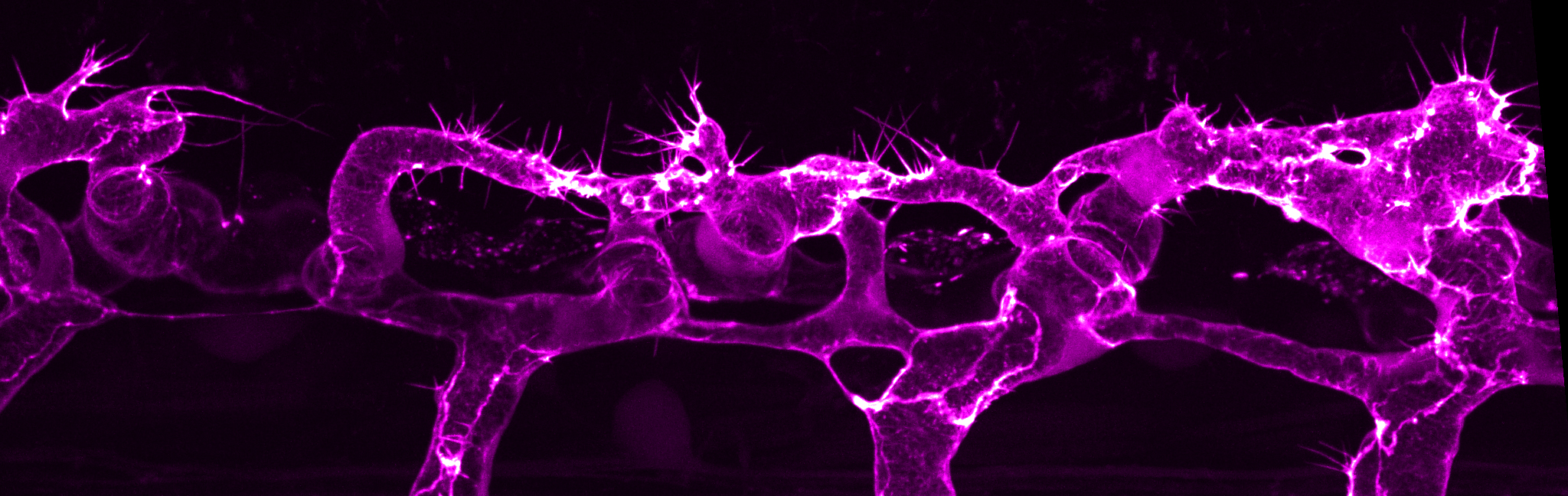
Blood vessels commonly form through the process of sprouting angiogenesis, a multicellular process that is tightly regulated in time and space. Sprouting angiogenesis begins with the formation of new vascular sprouts that emerge from pre-existing blood vessels. The vascular sprouts are composed of endothelial cells that migrate into the hypoxic tissue, with tip cells leading the way and stalk cells trailing. The polarised and coordinated migration of tip and stalk cells is mediated through cell-cell junctions, which act as sites of mechanocoupling to transmit force from one cell to another. Endothelial cells also undergo extensive cell shape changes necessary for cell rearrangements, anastomosis and lumen formation, which are cellular behaviours that are critical for generating a multicellular tubular vascular network.
While many key molecules and signaling pathways have been identified for vessel formation, there is still a poor understanding of how pro- and anti-angiogenic signals control endothelial cell behaviours to generate vessel tubes of precise size and shape and vascular networks of optimal pattern. Using the zebrafish as a genetic model system, we seek to unravel fundamental morphogenetic principles underlying vessel morphogenesis –from vessel sprouting to vessel remodelling. In particular, we are interested in understanding how intrinsic forces generated by actomyosin cytoskeleton and exogenous forces, such as pressurized blood flow, control endothelial cell mechanics to shape the vascular network.
Actin cytoskeletal dynamics and function during vessel morphogenesis
A major goal of the lab is to understand how actin cytoskeleton dynamics control endothelial cell behaviours during angiogenesis. We have previously demonstrated that actin cytoskeleton of different dynamics and localisation drive distinct cellular behaviours. The formation of linear actin bundles in filopodia is required for efficient endothelial cell migration and anastomosis (Phng et al., 2013). During transcellular lumen formation, localized actomyosin contractility at apical membranes retract blood pressure-induced inverse blebs to control lumen expansion (Gebala et al., 2016). At endothelial cell-cell junctions, formin-mediated actin cable assembly supports junction elongation and stabilizes vessels in a multicellular configuration (Phng et al., 2015). Future studies in the lab are aimed at understanding how cortical actin modulates membrane dynamics to shape endothelial cells and identifying molecules that regulate the formation of specialized actin structures in endothelial cells.
Endothelial cell mechanoresponse to haemodynamic forces
Once blood vessels become lumenized, endothelial cells are exposed to haemodynamic forces such as fluid shear stress and blood pressure. During lumen expansion, blood pressure locally deforms the apical membrane of endothelial cells to generate inverse blebs (Gebala et al., 2016). In turn, endothelial cells counteract the deforming forces by triggering a repair mechanism. Here, local and transient actomyosin activity is generated around the bleb cortex to retract the blebs, normalize apical membrane behaviour and allow controlled lumen expansion in the vessel. Recently, we further demonstrated that the organization of cortical actin cytoskeleton in perfused blood vessels is important in modulating endothelial cell mechanoresponse to haemodynamic forces. When excessive linear actin bundle formation is generated, the endothelial cell cortex becomes weaker and more deformable, leading to ectopic membrane blebbing (Kondrychyn et al., 2020). We next aim to understand how endothelial cells sense changes in haemodynamics to alter membrane and cellular behaviours.