Team Director
Li-Kun Phng
Ph.D.
Laboratory for Vascular Morphogenesis
LocationKobe / Developmental Biology Buildings
E-maillikun.phng@riken.jp
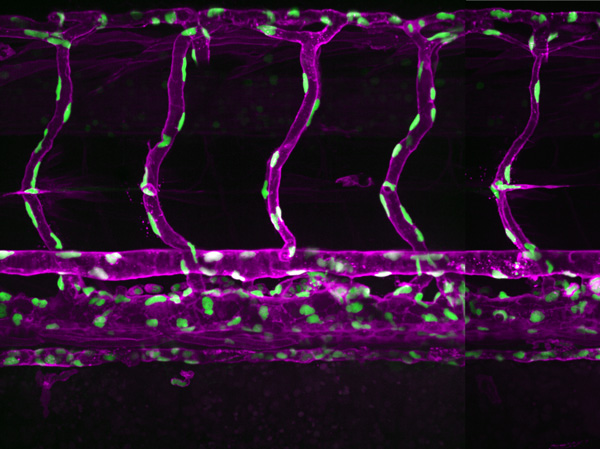
The establishment and maintenance of blood vessels is crucial throughout life. During development, blood vessels supply oxygen and nutrients to meet the demands of growing tissues. In the adult, they sustain the metabolic needs of organs to support homeostasis. Furthermore, new blood vessel formation is essential for tissue regeneration.
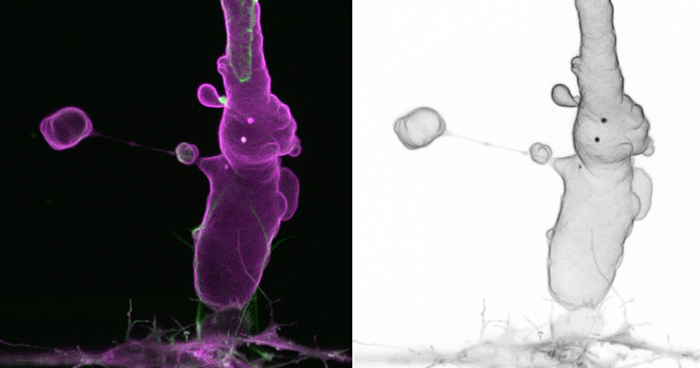
Our lab is interested in understanding the fundamental morphogenetic principles of how endothelial cells behave and coordinate with each other and the perivascular environment to generate a well-patterned network of blood vessels. Using the zebrafish as a model system, we seek to understand how endothelial cell shape plasticity is regulated to drive vessel formation and remodelling. We also aim to understand endothelial cell mechanoresponse to haemodynamic forces, in the control of vessel morphology. By unravelling the basic mechanisms of how endothelial cells build blood vessels of specific size and network pattern, we aim provide insights into how vascular malformations arise.
Research Theme
- How forces regulate endothelial cell shape changes and blood vessel morphogenesis.
- Mechanisms controlling actomyosin activity in endothelial cells.
- Molecular regulation of blood vessel lumen formation.
Selected Publications
Kondrychyn I, Kelly DJ, Taberner Carretero N, et al.
Marcksl1 modulates endothelial cell mechanoresponse to haemodynamic forces to control blood vessel shape and size.
Nature Communications
11, 5476 (2020)
doi: 10.1038/s41467-020-19308-5
Gebala V, Collins R, Geudens I, et al.
Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo.
Nature Cell Biology
18(4), 443-451 (2016)
doi: 10.1038/ncb3320
Phng LK, Gebala V, Bentley K, et al.
Formin-mediated actin polymerization at endothelial junctions is required for vessel lumen formation and stabilization.
Developmental Cell
32, 123-132 (2015)
doi: 10.1016/j.devcel.2014.11.017
Phng LK, Stanchi F, Gerhardt H.
Filopodia are dispensable for endothelial tip cell guidance.
Development
140, 4031-4040 (2013)
doi: 10.1242/dev.097352
Phng LK, Potente M, Leslie J D, et al.
Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis.
Developmental Cell
16, 70-82 (2009)
doi: 10.1016/j.devcel.2008.12.009
Hellström M, Phng LK, Hofmann J H, et al.
Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis.
Nature
445, 776-770 (2007)
doi: 10.1038/nature05571
Members

Team DirectorLi-Kun Phng
- likun.phng(at)riken.jp

Research ScientistIgor Kondrychyn, PhD
- igor.kondrychyn@riken.jp

Postdoctoral ResearcherYan Chen, PhD
- yan.chen@riken.jp

Postdoctoral ResearcherHaymar Wint, PhD
- haymarwint@riken.jp

Technical Staff ⅠJason Andrew Da Silva
- jason.dasilva@riken.jp

Technical Staff ⅠRajrishi Awadhesh Kumar
- rajrishi.kumar@riken.jp

Junior Research AssociateMingzhao Hu
- mingzhao.hu@riken.jp

AssistantEmi Taniguchi
- emi.taniguchi@riken.jp