Research | Laboratory for Nutritional Biology
Introduction
We eat almost every day from the time we are born until we die. In all animals, the process of development, growth, reproduction, and maintaining homeostasis requires constant intake of nutrients from the external environment. Thus, our healthspan is significantly influenced by the quality and quantity of the food we consume, but the detailed understanding of the molecular mechanisms is yet unknown. In our laboratory, we study the physiological functions of various nutrients at each life stage, including development, growth, reproduction, aging, and the adaptive mechanisms in response to nutritional deficiencies or excesses in animals. Our goal is to elucidate the molecular mechanisms that are affected by temporary or chronic disruptions in nutritional balance, impacting metabolic physiology, tissue homeostasis, feeding behavior, stress response, reproductive ability, and overall healthspan.
Traditionally, biomedical research has focused on how to cure diseases or prevent them. However, being healthy and not being sick are similar yet distinct concepts. We believe that organisms inherently possess the power to strive for health. By unraveling the mechanistic aspects of this power and revealing precise adaptive mechanisms to changes in nutrition, we aim to contribute to a fundamental understanding of life. We employ the fruit fly, Drosophila melanogaster, as a model organism for its short lifespan and excellent genetic and dietary tools. Fruit flies are cost-effective, easy to handle, and ethically less problematic, making them ideal for lifespan studies that require handling a large number of individuals. To understand universal and fundamental mechanisms of life, using simple organisms is advantageous. We aim to apply the knowledge gained from fruit flies to mammals and are also exploring research in non-human primates such as marmosets. Through these efforts, we strive to develop nutritional intervention methods that contribute to human health promotion, aging inhibition, and lifespan extension.


1. Elucidation of the mechanisms of lifespan extension through dietary restriction
The traditional Japanese phrase "Hara Hachi Bu", which emphasizes the idea that overeating is harmful, has been recognized. In many model organisms, restricting dietary intake has been shown to extend healthspan, and the elucidation of the underlying mechanisms has progressed. As a result, it is now understood that amino acids supplied from ingested proteins play a key role in many of the effects of dietary restriction. However, the comprehensive understanding of how amino acids are sensed within the body and what impact their depletion has on various cells composing the body remains unclear. Additionally, many questions remain unanswered regarding when and under what conditions amino acid levels and metabolic states change, as well as how the extension of healthspan through amino acid restriction occurs.
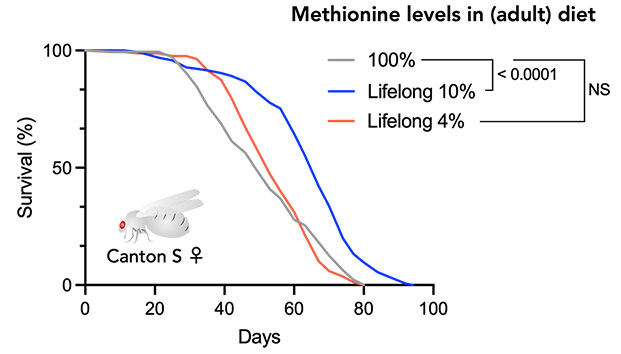
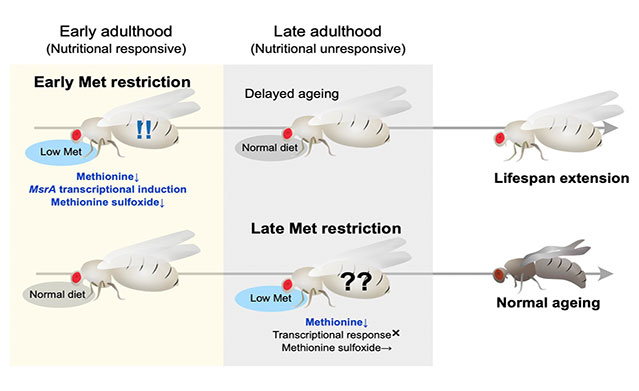
The essential amino acid, methionine, has been reported to be closely linked to lifespan, and it has been found that restricting methionine intake from the diet can extend lifespan. In our laboratory, we are using Drosophila to explore the mechanisms through which amino acids, including methionine, influence organismal lifespan and to clarify the effective range of these mechanisms. In this context, it has been discovered that the lifespan extension through methionine restriction is limited to the early stages of life and diminishes with aging (Kosakamoto, Obata et al., Nat Commun, 2023). Restricting methionine only during the early adulthood (the first 4 weeks of the 8–10 week lifespan of Drosophila) results in extended lifespan even when subsequently fed a normal diet. This indicates that the effects of dietary restriction depend on age. Numerous questions persist, such as why nutrient responses change with aging, how irreversible effects occur, and whether these findings apply to humans and mammals. Since amino acids other than methionine are also found to be associated with lifespan, we are analyzing their effects as well. Furthermore, it has been revealed that the intake of other nutrients affects methionine metabolism, and we are gradually unraveling the complex nutritional response mechanisms.
2. Specific Sensing of Nutrients and the Adaptive Mechanism to their Deficiency
Molecules (nutrients) that we intake daily through our diet are said to exceed 10,000 types, presenting a formidable challenge in ensuring their proper intake. However, it is believed that organisms possess mechanisms to specifically sense and adapt to each nutrient. For example, when a particular nutrient is deficient, its consumption and breakdown are suppressed, with an increase in biosynthesis if possible. Additionally, there is speculation that behavior (preference) may change to stimulate appetite and absorption of that nutrient. However, mechanisms for specific sensing of a diverse range of nutrients and their adaptive responses to deficiencies or excesses remain unknown.
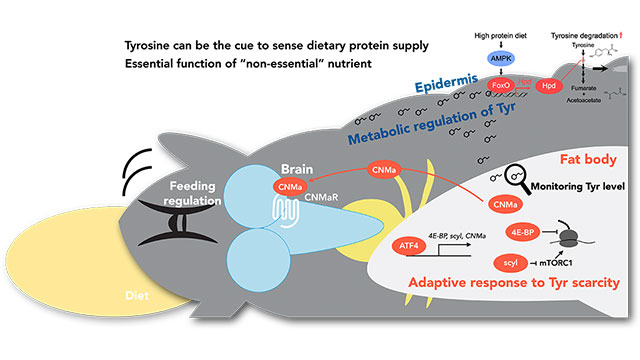
In our laboratory, we have previously elucidated adaptive mechanisms occurring when Drosophila larvae are subjected to a relatively mild protein-restricted diet during development. As a result, even under protein conditions with minimal negative impacts on growth, we observed a drastic reduction in translation and enhancement of feeding behavior, among other effects on energy and amino acid consumption. The mechanism revealed the significance of the non-essential amino acid "tyrosine," which has long been believed unnecessary to be obtained from the diet (Kosakamoto et al., Nat Metab, 2022). Tyrosine deficiency was found to control a transcription factor ATF4 in adipose tissue, triggering the adaptive response to a low-protein diet. Furthermore, we have discovered that tyrosine metabolism breakdown is strongly regulated in response to protein intake (Kosakamoto et al., Development, 2024). This tyrosine catabolism is activated through a transcription factor FoxO, which is shown to occur in epithelial, rather than adipose, tissues. The mechanisms governing the sensing and adaptation to a single nutrient vary across different organs, revealing an intricate and complex network. Our research extends beyond amino acids to include vitamins and minerals, and we are uncovering the existence of specific mechanisms for each nutrient.
3. Healthspan changes by early-life dietary environment
Nutritional and environmental stress during the developmental and early life stages are suggested to be memorized in organisms, potentially influencing the physiological state and aging process of adults. This hypothesis, known as DOHaD (Developmental Origins of Health and Disease), proposes the existence of mechanisms (nutritional programming) that "program" the physiological functions of adults and the risk of lifestyle diseases based on the environment during early life stages. However, DOHaD research is time-consuming due to the significant time gap between cause and consequence. Drosophila melanogaster, with its short life cycle, is suitable for analyzing how the healthspan is affected by the developmental environment.
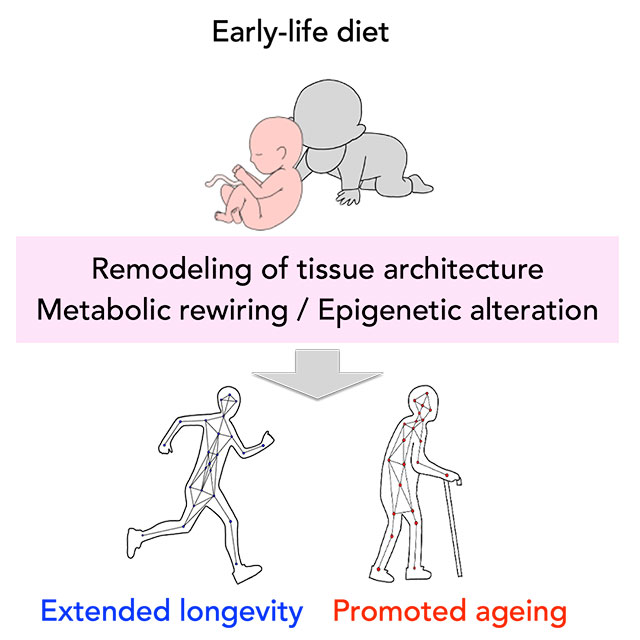
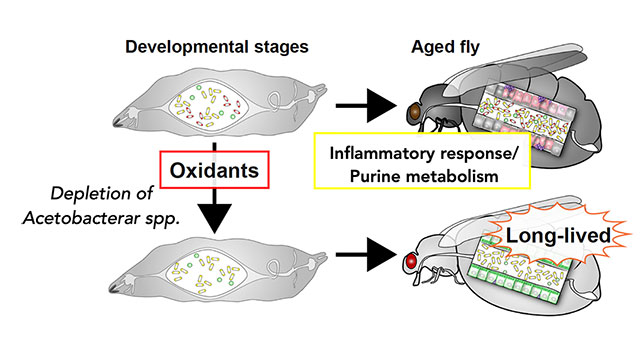
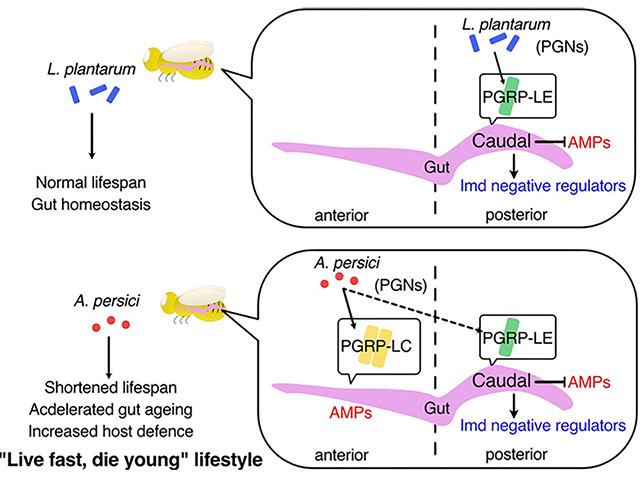
Previously, it was found that feeding Drosophila low concentrations of oxidants during development (larval stage) led to changes in metabolism and immunity, resulting in an extended lifespan (Obata et al., Nat Commun, 2018, Yamauchi et al., iScience, 2020). Analyzing the cause of this lifespan extension revealed an irreversible change in the composition of gut bacteria, particularly emphasizing the importance of the lifelong removal of Acetobacter genus bacteria. Recently, it was discovered that the peptidoglycan constituting the cell wall of Acetobacter genus bacteria shortens the healthspan of individuals through immune activation (Onuma et al., PLoS Genet, 2023). On the other hand, it was found that this bacterium enhances resistance to oral infection and oxidative stress, suggesting a lifestyle that promotes a "thick and short" life for Drosophila. Gut bacteria occupy a significant portion of our body and influence our health, but many aspects of their mechanism remain unknown. In our laboratory, we are working to elucidate the impact of immune activation by gut bacteria and the metabolites they produce on the host. Additionally, we are studying the functions of gut bacteria under various nutritional conditions.
Furthermore, it has been discovered that organismal lifespan can alter due to nutritional restriction during the early life stages. Given the uncertainty about how changes in protein levels not only affect growth but also influence subsequent healthspan, we are conducting analyses using Drosophila and higher animals to understand this mechanism better.