
Team Leader
Hiroshi Hamada
M.D., Ph.D.
Laboratory for Organismal Patterning
[Closed Mar. 2023]
E-mailhiroshi.hamada[at]riken.jp
Please replace [at] with @.
My lab studies how left-right asymmetries develop in the mouse embryo. In particular, we focus on two types of cilia that are required for left-right symmetry breaking: rotating cilia that generate leftward fluid flow, and immotile cilia that sense the fluid flow. We also study the role of maternal epigenetic regulators in development. We address these questions by integrating live imaging, structural biology, fluid dynamics and mathematical modeling.
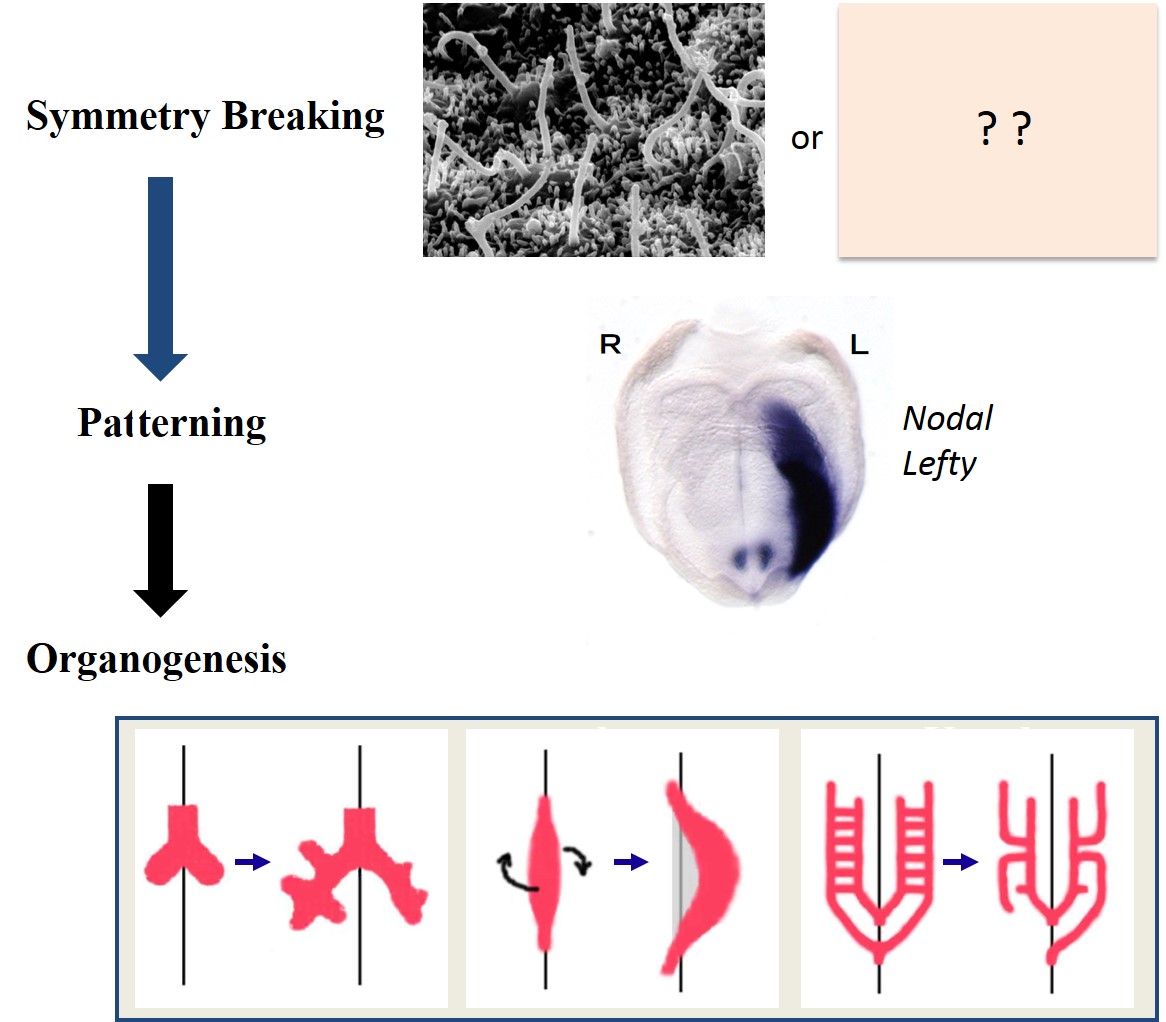
How left-right asymmetry is established in vertebrates
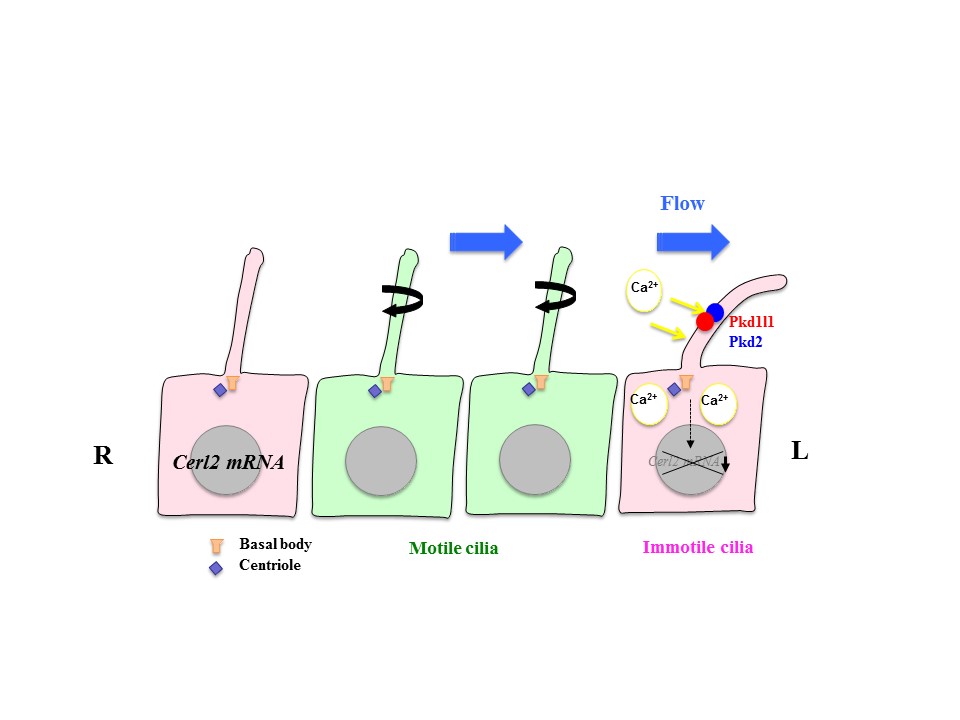
Role of motile and immotile cilia in left-right symmetry breaking
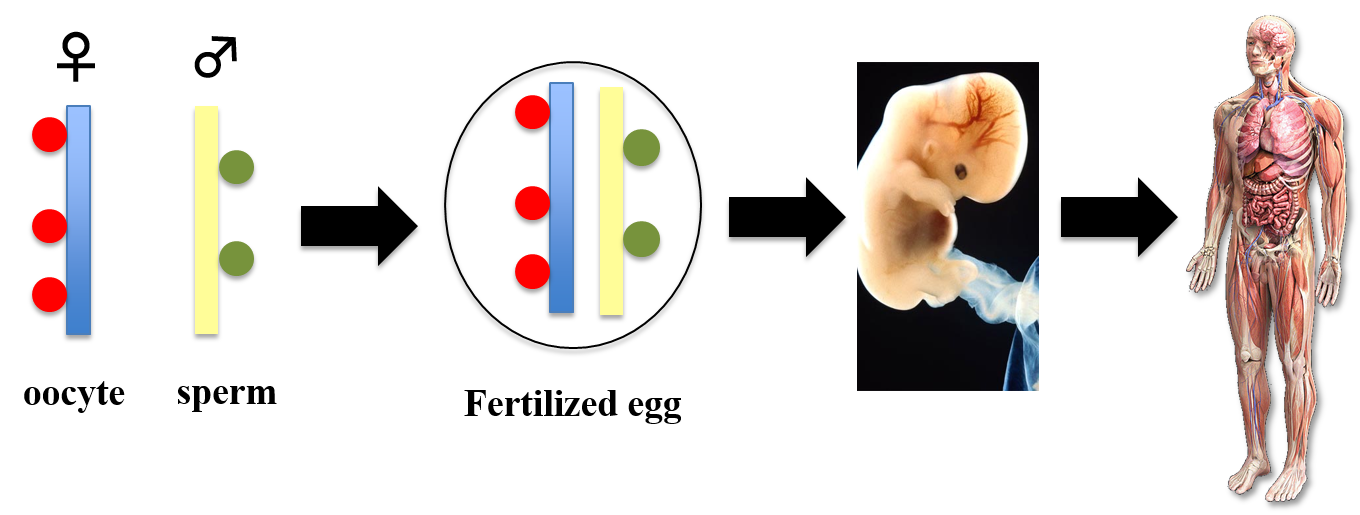
Maternal and paternal epigenome in development
Research Theme
- How motion pattern of node cilia is determined
- How immotile cilia sense fluid flow
- Role of maternal and paternal epigenetic factors in development
Selected Publications
Mizuno K, Shiozawa K, Katoh TA, et al.
Role of Ca2+ transients at the node of the mouse embryo in breaking of left-right symmetry.
Science Advances
6, eaba1195 (2020)
doi: 10.1126/sciadv.aba1195
Kajikawa E, Horo U, Ide T, et al.
Nodal paralogues underlie distinct mechanisms for visceral left-right asymmetry in reptiles and mammals.
Nature Ecology & Evolution
4, 261-269 (2020)
doi: 10.1038/s41559-019-1072-2
Nabeshima R, Nishimura O, Maeda T, et al.
Loss of Fam60a, a Sin3a subunit, results in embryonic lethality and is associated with aberrant methylation at a subset of gene promoters.
eLife
7, e36435 (2018)
doi: 10.7554/eLife.36435
Takaoka K, Nishimura H, Hamada H.
Both Nodal signaling and stochasticity select for prospective distal visceral endoderm in mouse embryos.
Nature Communications
8, 1492 (2017)
doi: 10.1038/s41467-017-01625-x
Minegishi K, Hashimoto M, Ajima R, et al.
A Wnt5 activity asymmetry and intercellular signaling polarize node cells for breaking left-right symmetry in the mouse embryo.
Developmental Cell
40, 439-452 (2017)
doi: 10.1016/j.devcel.2017.02.010
Shinohara K, Chen D, Nishida T, et al.
Absence of radial spokes in mouse node cilia is required for rotational movement but confers ultrastructural instability as a trade-off.
Developmental Cell
35, 236-246 (2015)
doi: 10.1016/j.devcel.2015.10.001
Nakamura T, Saito D, Kawasumi A, et al.
Fluid flow and interlinked feedback loops establish left-right asymmetric decay of Cerl2 mRNA in the mouse embryo.
Nature Communications
3, 1322 (2012)
doi: 10.1038/ncomms2319
Yoshiba S, Shiratori H, Kuo IY, et al.
Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2.
Science (New York, N.Y.)
338(6104), 226-31 (2012)
doi: 10.1126/science.1222538
News
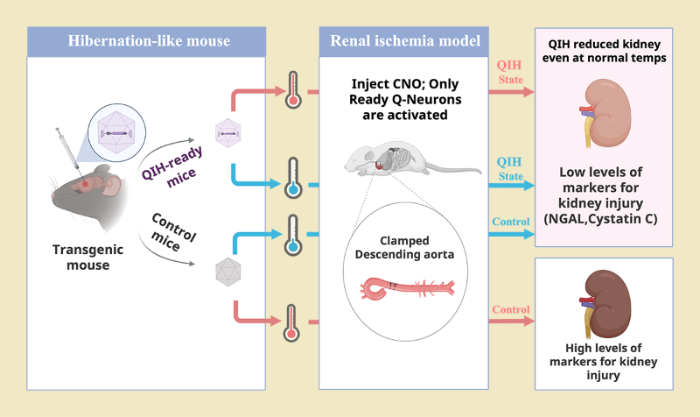
Nov. 18, 2022 Research
Inducing hibernation-like state in mice can protect organs during heart surgery

May 17, 2022 BDR News
Hiroshi Hamada elected as a Foreign Member of the Royal Society
Oct. 18, 2021 Research
mRNA degradation induced by fluid flow breaks left–right symmetry in vertebrates

Nov. 11, 2020 BDR News
Team Leader Hamada Receives Hyogo Prefecture Science Award
Oct. 12, 2018 Research
Gene Fam60a found to play a key role in the developing embryo



