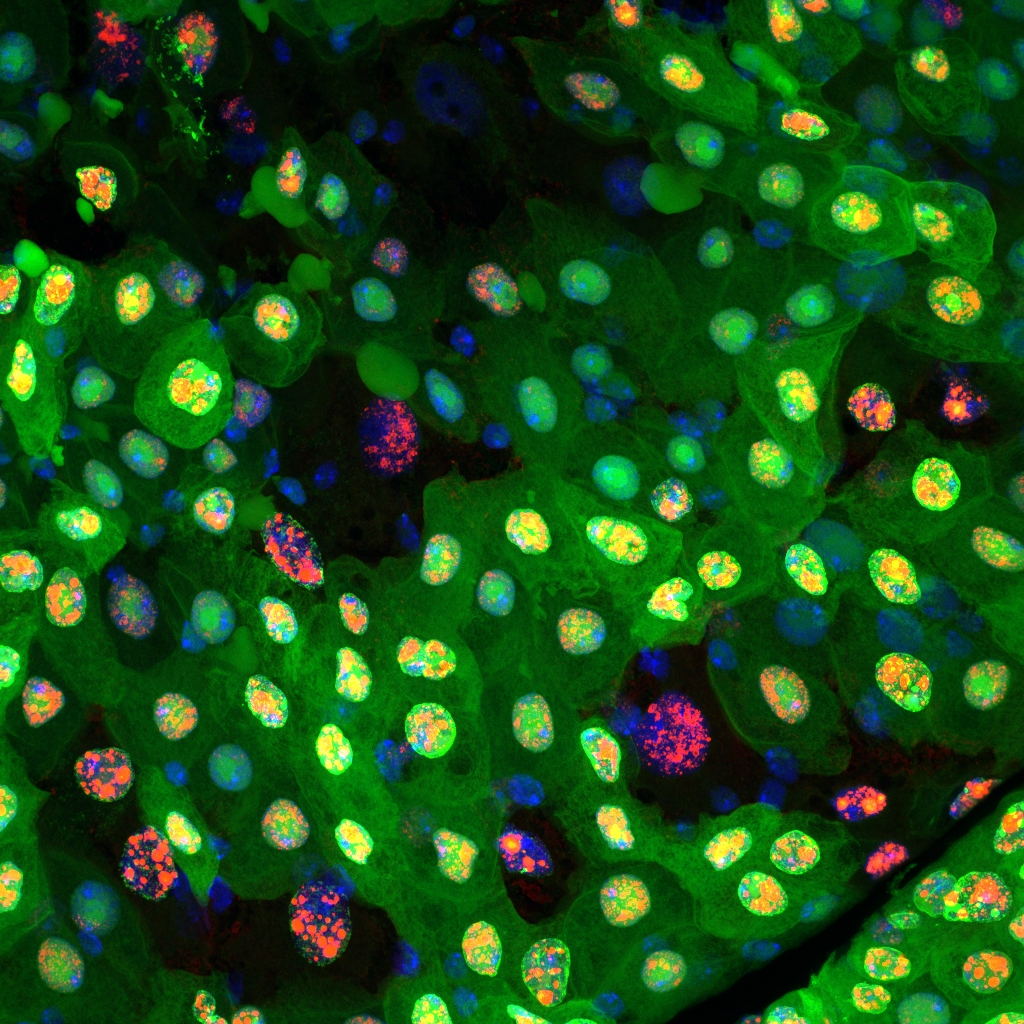
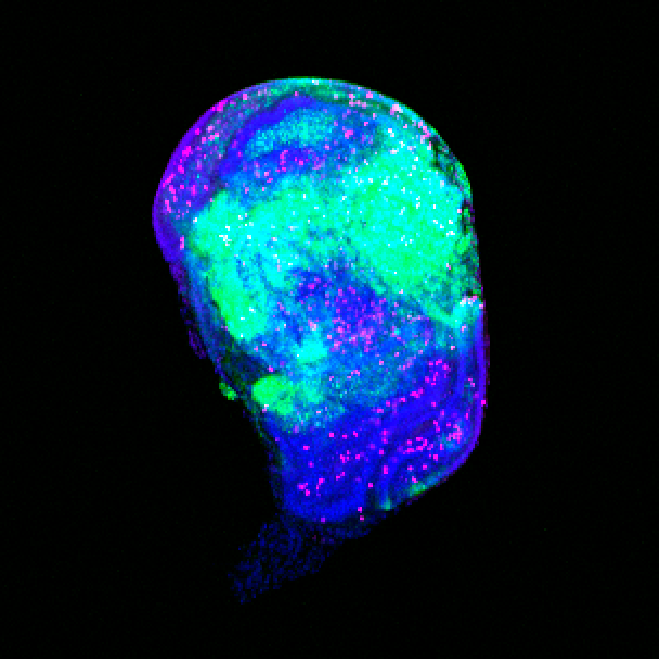
Team Director
Sa Kan Yoo
MD, Ph.D.
Laboratory for Homeodynamics
LocationKobe / Developmental Biology Buildings
E-mailsakan.yoo[at]riken.jp
Please replace [at] with @.
Living organisms can cope with disruption of homeostasis such as injury and disease to a certain degree. This results in restoration of steady state homeostasis, death of affected tissues or ultimate organismal death. The outcome depends on the type of insults, species of animals and maturation of tissues. While the process is well recognized, we still do not know the mechanisms defining responses to disruption of homeostasis in different organs and species. Our research focuses on fundamental questions targeting major disruption of homeostasis in organisms:
How does oncogenic stress by cancer affect animals?
What is the mechanism of aging?
How do animals cope with malnutrition?
Why are tissue stem cells perseverant?
How do cell death and stem cell proliferation coordinate?
To do this, we mainly utilize unrivaled genetics of the fruit fly, Drosophila melanogaster.
Selected Publications
Sakizli U, Takano T, Yoo SK.
GALDAR: A genetically encoded galactose sensor for visualizing sugar metabolism in vivo
PLoS biology
22(3), e3002549 (2024)
doi: 10.1371/journal.pbio.3002549
Sulekh S, Ikegawa Y, Naito S, et al.
A feedback loop that drives cell death and proliferation and its defect in intestinal stem cells
Life Science Alliance
7(4), (2024)
doi: 10.26508/lsa.202302238
Nishida H, Albero AB, Onoue K, et al.
Necrosensor: a genetically encoded fluorescent sensor for visualizing necrosis in Drosophila
Biology Open
13(1), (2024)
doi: 10.1242/bio.060104
Okada M, Takano T, Ikegawa Y, et al.
Oncogenic stress-induced Netrin is a humoral signaling molecule that reprograms systemic metabolism in Drosophila
The EMBO Journal
(2023)
doi: 10.15252/embj.2022111383
Ikegawa Y, Combet C, Groussin M, et al.
Evidence for existence of an apoptosis-inducing BH3-only protein, sayonara, in Drosophila
The EMBO Journal
42(8), e110454 (2023)
doi: 10.15252/embj.2021110454
Ciesielski HM, Nishida H, Takano T, et al.
Erebosis, a new cell death mechanism during homeostatic turnover of gut enterocytes
PLoS Biology
20(4), e3001586 (2022)
doi: 10.1371/journal.pbio.3001586
Nishida H, Okada M, Yang L, et al.
Methionine restriction breaks obligatory coupling of cell proliferation and death by an oncogene Src in Drosophila.
eLife
10, e59809 (2021)
doi: 10.7554/eLife.59809
Sasaki A, Nishimura T, Takano T, et al.
white regulates proliferative homeostasis of intestinal stem cells during ageing in Drosophila.
Nature metabolism
3(4), 546-557 (2021)
doi: 10.1038/s42255-021-00375-x
Yoo SK, Pascoe H, Pereira T, et al.
Plexins function in epithelial repair in both Drosophila and zebrafish.
Nature Communications
7, 12282 (2016)
doi: 10.1038/ncomms12282
Yoo SK, Freisinger C, LeBert D, Huttenlocher A.
Early redox, Src family kinase and calcium signaling integrate wound responses and tissue regeneration in zebrafish.
Journal of Cell Biology
199, 225-34 (2012)
doi: 10.1083/jcb.201203154
Yoo SK, Lam PY, Eichelberg M, et al.
The role of microtubules in neutrophil polarity and migration in live zebrafish.
Journal of Cell Science
125, 5702-10 (2012)
doi: 10.1242/jcs.108324
Yoo SK, Starnes TW, Deng Q, Huttenlocher A.
Lyn is a redox sensor that mediates leukocyte wound attraction in vivo .
Nature
480, 109-12 (2012)
doi: 10.1038/nature10632
Deng Q, Yoo SK, Cavnar P, et al.
Dual roles for Rac2 in neutrophil motility and retention in zebrafish hematopoietic tissues.
Developmental Cell
21, 735-45 (2011)
doi: 10.1016/j.devcel.2011.07.013
Yoo SK, Huttenlocher A.
Spatiotemporal photolabeling of neutrophils during induction and resolution of inflammation in zebrafish.
Journal of Leukocyte Biology
89, 661-667 (2011)
doi: 10.1189/jlb.1010567
Walters KB, Green JM, Surfus JC, et al.
Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome.
Blood
116, 2803-2811 (2010)
doi: 10.1182/blood-2010-03-276972
Yoo SK, Deng Q, Cavnar P, et al.
Differential regulation of protrusion and polarity by PI(3)K during neutrophil motility in live zebrafish.
Developmental Cell
18, 226-236 (2010)
doi: 10.1016/j.devcel.2009.11.015
Yoo SK, Huttenlocher A.
Innate immunity: wounds burst H2 O2 signals to leukocytes.
Current Biology
19, 553-5 (2009)
doi: 10.1016/j.cub.2009.06.025
Nishita M, Yoo SK, Nomachi A, et al.
Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration.
Journal of Cell Biology
175, 555-62 (2006)
doi: 10.1083/jcb.200607127
News
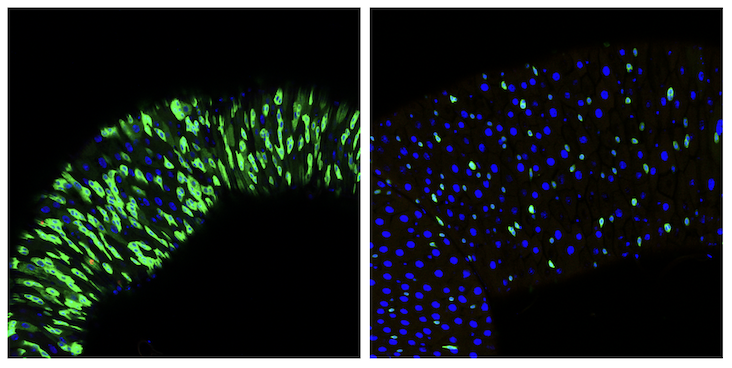
Sep. 10, 2024 Research
How aging affects stem cells: A fly's tale
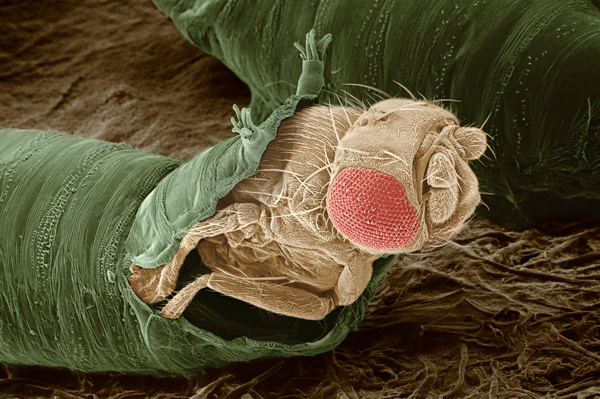
Oct. 25, 2023 Research
Signaling molecule from cancer cells induces metabolic changes to fruit fly larvae

May 8, 2023 Research
Flies aren’t freaks when it comes to cell death
Oct. 25, 2022 Research
A cell death find changes the gut paradigm
Apr. 26, 2022 Research
Death in darkness: a new type of cell death discovered in fly guts

Sep. 24, 2021 BDR News
RIKEN People
Stress and stability in cells and tissues

Jun. 24, 2021 Research
Age-related stem cell dysfunction linked to eye-color gene
Apr. 27, 2021 Research
Dietary amino acid determines the fate of cancer cells