
Team Leader*
Masatoshi Takeichi
Ph.D.
Laboratory for Cell Adhesion and Tissue Patterning
[Closed Mar. 2020]
*Current positionSenior Visiting Scientist, BDR
RIKEN Honorary Scientist
Animal cells organize into tissues with complex architecture. Our lab is exploring the molecular mechanisms by which individual cells assemble into a tissue-specific multicellular structure, such as epithelial sheets and neural networks. We are also interested in the molecular basis of how tissue architecture is disrupted during carcinogenesis, a process that is thought to accelerate the metastasis of cancer cells. For these studies, we are focusing on the roles played by cell-cell adhesion molecules, the cadherin family of adhesion molecules in particular, as these are known to be indispensable for tissue construction. Our current studies are divided into three categories:
1) Cell-cell adhesion is a dynamic process, and this feature of cell adhesion is implicated in various kinds of cell behavior including tissue morphogenesis and cancer invasion. A growing body of evidence suggests that cadherins cooperate with cytoskeletal and/or motility machineries, such as actin regulators, non-muscle myosins, and Rho GTPases, via the catenins that bind the cadherin cytoplasmic domain. We are therefore studying the molecular mechanisms underlying the crosstalk between catenins and such cytoskeletal systems, and their roles in epithelial junction formation as well as in its disruption.
2) Recent studies from our laboratory have shown that the catenin-dependent regulation of cytoskeletal proteins also regulates the front-rear polarity of migrating cells, independently of cadherin or cell adhesion. We are continuing this study, and will reveal the roles of the catenin-dependent regulation of cell migration in morphogenetic or pathogenic cell behavior.
3) In addition, we have been analyzing the functions of microtubule minus end-associated proteins, Nezha/CAMSAPs. These proteins regulate microtubule assembly and dynamics, as well as intracellular architecture. We are exploring the roles of these molecules in cellular morphogenesis, such as polarized epithelial formation and axon growth, with the aim of uncovering novel functions of non-centrosomal microtubules.
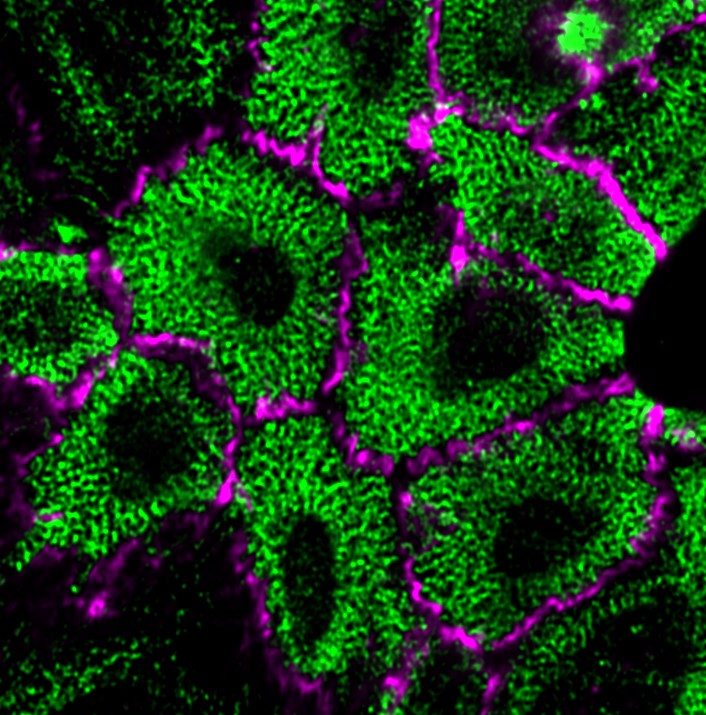
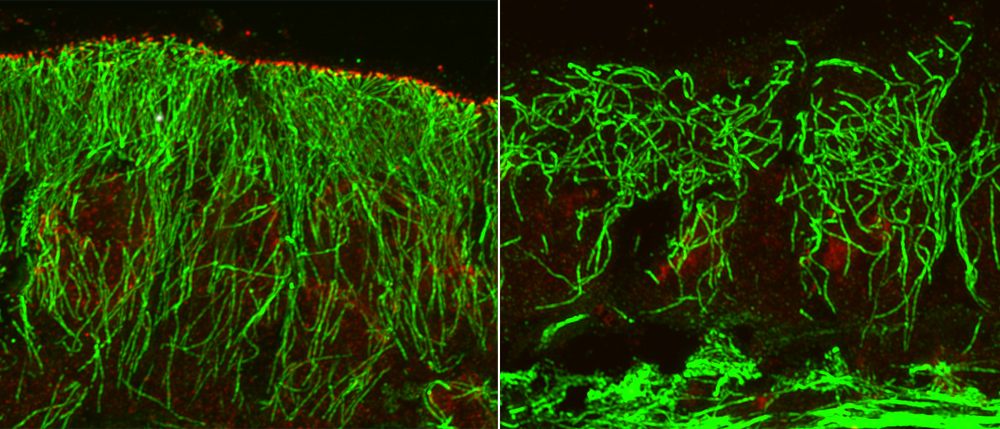
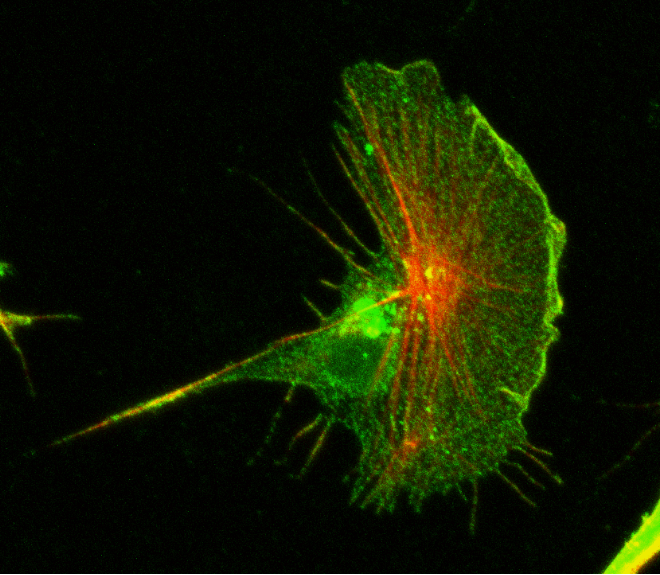
Research Theme
- Regulation of cell adhesion and migration by cadherins and catenins
- Mechanisms to cause adhesive defects in cancer cells
- Regulation of epithelial and neural morphogenesis by CAMSAPs, microtubule regulators
Selected Publications
Pongrakhananon V, Saito H, Hiver S, et.al.
CAMSAP3 maintains neuronal polarity through regulation of microtubule stability.
Proceedings of the National Academy of Sciences 115(39). 9750-9755 (2018) doi: 10.1073/pnas.1803875115
Ito S, Okuda S, Abe M, et al.
Induced cortical tension restores functional junctions in adhesion-defective carcinoma cells.
Nature Communications 8. 1834 (2017) doi :10.1038/s41467-017-01945-y
Vassilev V, Platek A, Hiver S, et al.
Catenins Steer Cell Migration via Stabilization of Front-Rear Polarity.
Developmental Cell 43. 463–479 (2017) doi:10.1016/j.devcel.2017.10.014
Nishimura T, Ito S, Saito H, et al.
DAAM1 stabilizes epithelial junctions by restraining WAVE complex-dependent lateral membrane motility.
Journal of Cell Biology 215. 559–573 (2016) doi:10.1083/jcb.201603107
Toya M, Kobayashi S, Kawasaki M, et al.
CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells.
Proceedings of the National Academy of Sciences of the United Sciences of America 113. 332–337 (2016) doi:10.1073/pnas.1520638113
Tsukasaki Y, Miyazaki N, Matsumoto A, et al.
Giant cadherins Fat and Dachsous self-bend to organize properly spaced intercellular junctions.
Proceedings of the National Academy of Sciences of the United States of America 111. 1601–6 (2014) doi: 10.1073/pnas.1418990111
Hayashi S, Inoue Y, Kiyonari H, et al.
Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts.
Developmental Cell 30. 673–687 (2014) doi:10.1016/j.devcel.2014.07.015





