
Team Leader
Fumio Matsuzaki
Ph.D.
Laboratory for Cell Asymmetry
[Closed Mar. 2023]
Our group explores the mechanisms underlying the organization of cells into highly ordered structures in the developing brain. During brain development, neural stem cells generate a large number of neurons and glia of different fates at appropriate points in time; the framework and size of the brain depend on the spatiotemporal behavior of neural stem cells, which are highly dynamic in their modes of division and gene expression. Using invertebrate (Drosophila ) and vertebrate (mouse and ferret) model systems, we focus our study on the programs by which behaviors of neural stem cells are controlled and brain development is governed.
Drosophila neural stem cells, called neuroblasts, provide an excellent model system for the investigation of fundamental aspects of asymmetric division, a process essential to the generation of cells of divergent type during proliferation. We have been investigating mechanisms controlling asymmetric divisions, including the cell polarity and spindle orientation. We also extend our research scope to understand how neurogenesis is controlled in tissue space depending on the environments that surround the nervous system. We recently identified an extrinsic mechanism that controls the orientation of division (cell polarity) in neuroblasts relative to the overriding ectoderm (Yoshiura et al., 2012), which determines the orientation of neural tissue growth.
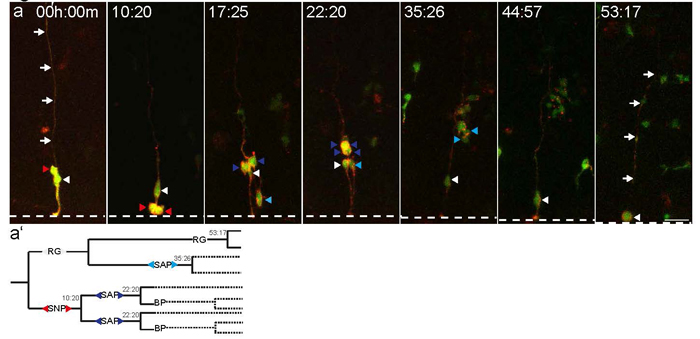
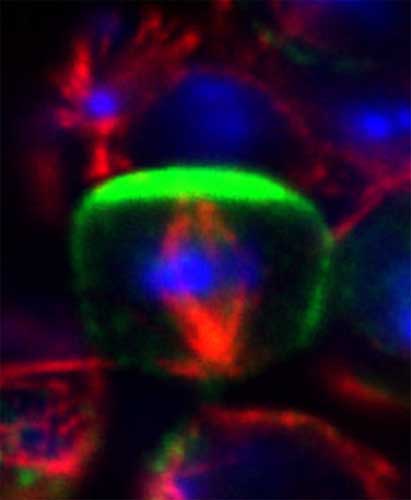
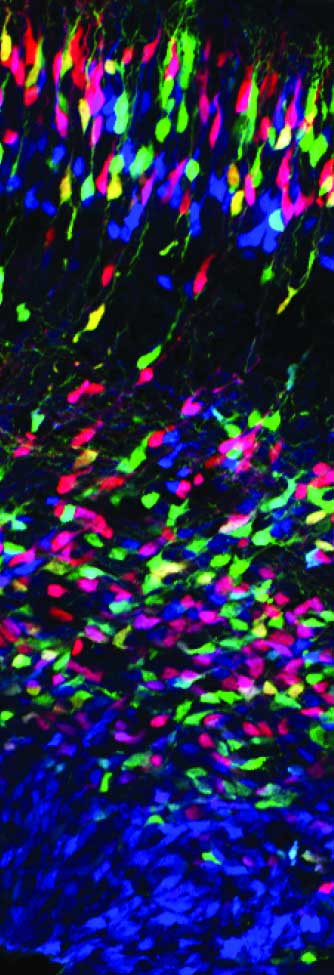
The vertebrate brain evolved rapidly, resulting in an expansion of the size of the brain, which comprises a larger number of neurons arranged in a vastly more complex functional network than that in invertebrate. Neural stem cells typically adopt three states—proliferative (symmetrically dividing), neurogenic (asymmetrically dividing), and resting—and undergo transitions among the states, on which the basic organization of the brain depend. We are investigating mechanisms that determine the individual states of neural stem cells, and control transitions between states in mouse as well as mechanisms for generating neural progenitor cell diversity (see figure). We recently discovered a novel transition in the division mode in the developing mouse cortex from radial glia (typical neural stem cells with the epithelial structure) to translocating neural stem cells, basal radial glia (Shitamukai et al., 2011), which become a major population of neural stem cells in mammals with gyrencephalic brains, such as primates and ferrets. We are investigating the mechanisms that underlie the formation, maintenance, and expansion of these neural stem cells, by using model mice that produce large numbers of basal radial glia as well as ferrets as a model forming the complex brain (Tsunekawa et al., 2016).
Research Theme
- Genetic programs of neural development and maintenance
- Asymmetric division of neural stem cells
Selected Publications
Fujita I, Shitamukai A, Kusumoto F, et al.
Endfoot regeneration restricts radial glial state and prevents translocation into the outer subventricular zone in early mammalian brain development.
Nature Cell Biology
22, 26-37 (2020)
doi: 10.1038/s41556-019-0436-9
Kono K, Yoshiura S, Fujita I, et al.
Reconstruction of Par-dependent polarity in apolar cells reveals a dynamic process of cortical polarization.
eLife
8, e45559 (2019)
doi: 10.7554/eLife.45559.001
Kawaue T, Shitamukai A, Nagasaka A, et al.
Lzts1 controls both neuronal delamination and outer radial glial-like cell generation during mammalian cerebral development.
Nature communications
10(1), 2780 (2019)
doi: 10.1038/s41467-019-10730-y
Suzuki K, Tsunekawa Y, Hernandez-Benitez R, et al.
In vivo genome editing via CRISPR-Cas9 mediated homology-independent targeted integration.
Nature
540, 144-149 (2016)
doi: 10.1038/nature20565
Tsunekawa Y, Terhune R K, Fujita I, et al.
Developing a de novo targeted knock-in method based on in utero electroporation into the mammalian brain.
Development
143, 3216-3222 (2016)
doi: 10.1242/dev.136325
Okamoto M, Miyata T, Konno D, et al.
Cell cycle–independent transitions in temporal identity of mammalian neural progenitor cells.
Nature Communications
7, 11349 (2016)
doi: 10.1038/ncomms11349
Matsuzaki F, Shitamukai A.
Cell Division Modes and Cleavage Planes of Neural Progenitors during Mammalian Cortical Development.
Cold Spring Harbor perspectives in biology
7(9), a015719 (2015)
doi: 10.1101/cshperspect.a015719
Pilz GA, Shitamukai A, Reillo I, et al.
Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type.
Nature communications
4, 2125 (2013)
doi: 10.1038/ncomms3125
Yoshiura S, Ohta N, Matsuzaki F.
Tre1 GPCR signaling orients stem cell divisions in the Drosophila central nervous system.
Developmental Cell
22, 79-91 (2012)
doi: 10.1016/j.devcel.2011.10.027
News
Mar. 13, 2020 Research
Stem cells exert tight control over the timing of brain development
Jul. 26, 2019 Research
A new model for exploring cell polarity

Jun. 26, 2019 Research
Unified control of neuronal delamination and outer radial glial cell generation during mammalian cerebral development (PDF file : 204KB)



