
Team Director
Hironobu Fujiwara
Ph.D.
Laboratory for Tissue Microenvironment
Location Kobe / Developmental Biology Buildings
E-mailhironobu.fujiwara[at]riken.jp
Please replace [at] with @
We study how tissue stem cells and their microenvironments interact to regulate organ development, homeostasis and regeneration using mammalian skin as a model.
Tissue stem cells are specialized cells with the capacity for long-term self-renewal and differentiation into multiple cell types, and play a central role in organ development, homeostasis and regeneration. The behaviour and fate of stem cells are regulated by signals from their microenviroment, called the ‘niche’, which is composed of various components, such as extracellular matrix, growth factors and surrounding cells. However, it remains unclear how stem cells and their niches are induced during development, how they communicate with each other, and how these communication networks are altered during tissue regeneration and repair.
We have recently elucidated the developmental origin of hair follicle epithelial stem cells. Stem cells are originated from the outermost ring region of a 2D concentric pre-pattern in the hair placode, and are induced into their future stem cell niche through close coupling with 3D tissue deformation and expansion. Our studies and others have also shown that stem cells do not simply respond to signals from the niche, rather they play an integral role in creating and communicating with their niches. We are applying and developing new imaging tools and techniques, and combine them with single-cell transcriptomics to help us study dynamic interactions between stem cells and their niches. Stem cell dysfunction causes a wide variety of diseases, such as birth defects and cancer, and thus understanding of stem cell-niche communications has huge implications in future medical advances.
Research Theme
- Stem cell induction and maintenance, and its extrinsic regulation in the skin
- Extracellular matrix heterogeneity in skin development and regeneration
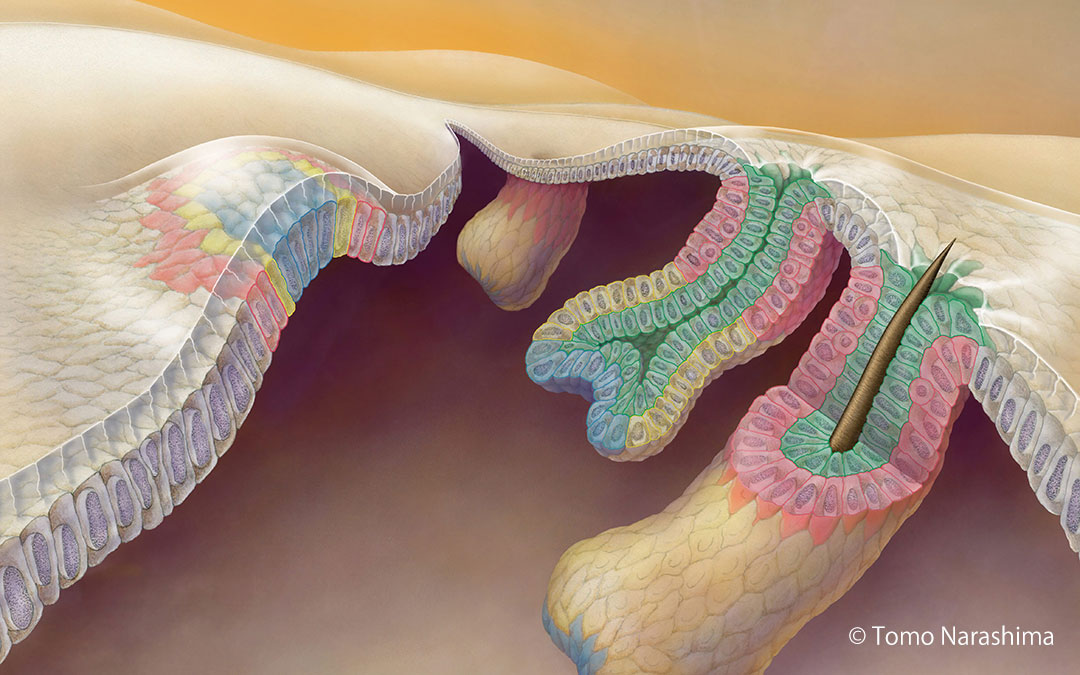
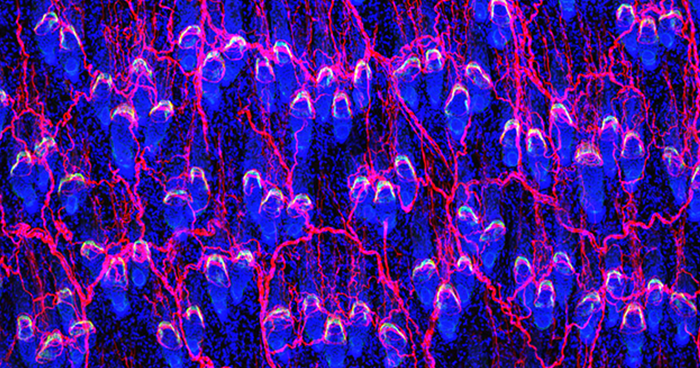
Hair follicles grow outward, like a telescopic antenna (prospective stem cells shown in pink).
Selected Publications
Wuergezhen D, Gindroz E, Morita R, et al.
An eGFP-Col4a2 mouse model reveals basement membrane dynamics underlying hair follicle morphogenesis.
Journal of Cell Biology
224(2), e202404003 (2025)
doi: 10.1083/jcb.202404003
Fujiwara H.
Dynamic duo: Cell-extracellular matrix interactions in hair follicle development and regeneration.
Developmental Biology
516, 20-34 (2024)
doi: 10.1016/j.ydbio.2024.07.012
Morita R, Fujiwara H.
Tracing the developmental origin of tissue stem cells.
Development, Growth & Differentiation
64(9), 566-576 (2022)
doi: 10.1111/dgd.12816
Morita R, Sanzen N, Sasaki H, et al.
Tracing the origin of hair follicle stem cells.
Nature
594, 547-552 (2021)
doi: 10.1038/s41586-021-03638-5
Tsutsui K, Machida H, Nakagawa A, et al.
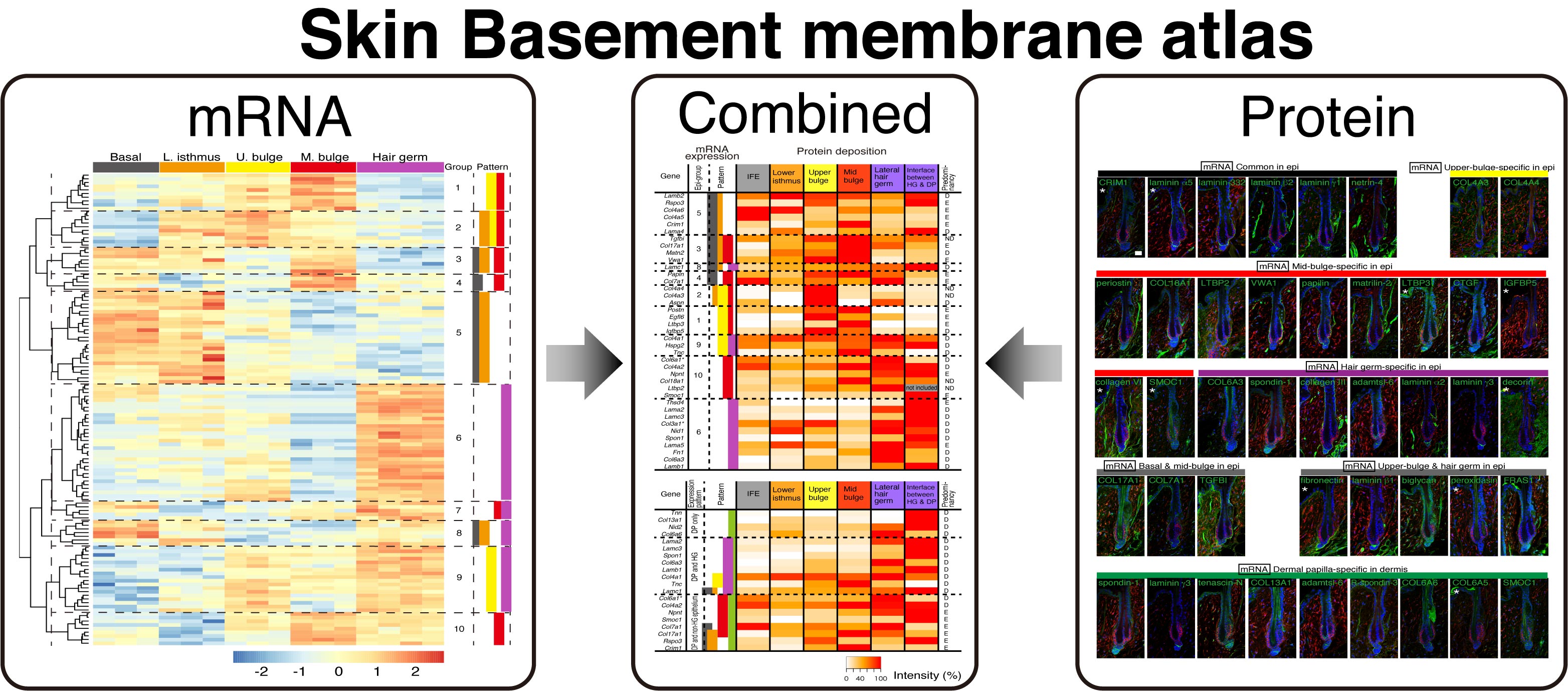
Mapping the molecular and structural specialization of the skin basement membrane for inter-tissue interactions.
Nature communications
12(1), 2577 (2021)
doi: 10.1038/s41467-021-22881-y
Fujiwara H, Tsutsui K, Morita R, et al.
Multi-tasking epidermal stem cells: Beyond epidermal maintenance.
Development, Growth & Differentiation
60(9), 531-541 (2018)
doi: 10.1111/dgd.12577
Cheng CC, Tsutsui K, Taguchi T, et al.
Hair follicle epidermal stem cells define a niche for tactile sensation.
eLife
7, e38883 (2018)
doi: 10.7554/eLife.38883
Donati G, Proserpio V, Lichtenberger B M, et al.
Epidermal Wnt/beta-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors.
Proceedings of the National Academy of Sciences of the United States of America
111, E1501-9 (2014)
doi: 10.1073/pnas.1312880111
Fujiwara H, Ferreira M, Donati G, et al.
The basement membrane of hair follicle stem cells is a muscle cell niche.
Cell
144, 577-89 (2011)
doi: 10.1016/j.cell.2011.01.014
Watt F M, Fujiwara H.
Cell-extracellular matrix interactions in normal and diseased skin.
Cold Spring Harbor Perspectives in Biology
3(4), a005124 (2011)
doi: 10.1101/cshperspect.a005124
メンバー

Hironobu Fujiwara
Team Director
Kohei Omachi
Special Postdoctoral Researcher
Momoko Deguchi
JSPS PD Researcher
Eleanor Louise Sheekey
Postdoctoral Researcher
Asako Nakagawa
Technical Staff II
Noriko Ban
Technical Staff II
Kokoro Kaiden
Junior Research Associate
Shuyu Dong
International Program Associate
Xiaomeng Yu
International Program Associate
Yutaro Imafuku
Student Trainee
Haruka Matsuzoe
Student Trainee
Tomoya Kawabata
Student Trainee
Hiroki Machida
Research Part-time Worker I
Hiroko Sasaki
Temporary Staffing
News

Oct. 8, 2021 BDR News
Dive into BDR's intriguing research
Everything is Done Manually
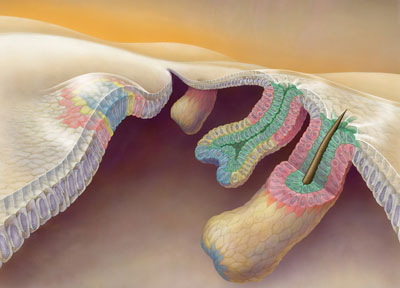
Oct. 6, 2021 Research
A telescopic model of the development of hair follicles
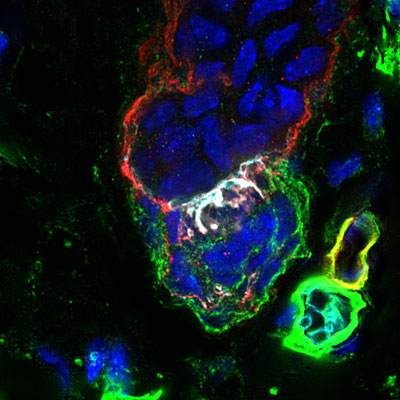
Jul. 20, 2021 Research
Basement membrane underpins tissue interactions in the skin

Jul. 1, 2020 BDR News
Ritsuko Morita awarded Shiseido Female Researcher Science Grant
Jan. 18, 2019 Research
Hair follicle stem cells secrete protein that controls the skin’s sense of touch




