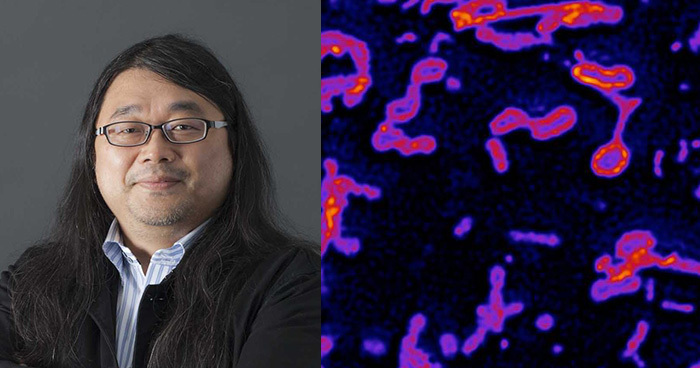
Team Director
Yasushi Okada
M.D., Ph.D.
Laboratory for Cell Polarity Regulation
Location Kobe / Developmental Biology Buildings
E-maily.okada@riken.jp
Although there have been big advances in molecular biology and structural biology, we still cannot answer the very basic question “What is life?” We are approaching this question by imaging cellular processes at the single molecule level in living cells. For this purpose, we develop new technologies by ourselves ranging from microscope optics to probes. Using these new, original technologies, we are mainly focusing on the movement or the trafficking of proteins and other molecules in living cells, especially neurons. For example, we have been working on the regulation of the axonal transport in neurons. Motor proteins transport a variety of elements inside the cell. In fact, so important is this transport that it is not an exaggeration to describe it as the lifeline of a cell. When a motor malfunctions, the cell’s internal navigation system becomes disabled so that transport is compromised. We are studying the navigation system by directly observing transport using new imaging techniques and the motor protein kinesin KIF5, a key regulator for axonal development, as our model.
Despite neurons extending a large number of projections, only one becomes an axon. Recently, we have discovered that the structures of the microtubules on which kinesins travel in dendrites and the axon are different. KIF5 can recognize the structural difference between these microtubules and therefore be used to determine which neural projections become the axon and which become dendrites.
Fluorescent live cell imaging of axonal transport

3D time-lapse super-resolution imaging of ER
Research Theme
- Molecular mechanisms of intracellular transport and its regulation
- Development of microscope optics and probes for single molecule imaging and super-resolution imaging
- Visualization of cellular processes by single molecule imaging and super-resolution live cell imaging
Selected Publications
Fujioka Y, Alam JM, Noshiro D, et al.
Phase separation organizes the site of autophagosome formation.
Nature
578(7794), 301-305 (2020)
doi: 10.1038/s41586-020-1977-6
Kono K, Yoshiura S, Fujita I, et al.
Reconstruction of Par-dependent polarity in apolar cells reveals a dynamic process of cortical polarization.
eLife
8, e45559 (2019)
doi: 10.7554/eLife.45559.001
Wang C, Taki M, Sato Y, et al.
A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae.
Proceedings of the National Academy of Sciences of the United States of America
116(32), 15817-15822 (2019)
doi: 10.1073/pnas.1905924116
Shima T, Morikawa M, Kaneshiro J, et al.
Kinesin-binding-triggered conformation switching of microtubules contributes to polarized transport.
The Journal of cell biology
217(12), 4164-4183 (2018)
doi: 10.1083/jcb.201711178
Hayashi K, Tsuchizawa Y, Iwaki M, Okada Y.
Application of the fluctuation theorem for noninvasive force measurement in living neuronal axons.
Molecular biology of the cell
29(25), 3017-3025 (2018)
doi: 10.1091/mbc.E18-01-0022
Grzybowski M, Taki M, Senda K, et al.
A Highly Photostable Near-Infrared Labeling Agent Based on a Phospha-rhodamine for Long-Term and Deep Imaging.
Angewandte Chemie (International ed. in English)
57(32), 10137-10141 (2018)
doi: 10.1002/anie.201804731
Takai A, Nakano M, Saito K, et al.
Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging.
Proceedings of the National Academy of Sciences of the United States of America
112(14), 4352-4356 (2015)
doi: 10.1073/pnas.1418468112
Hayashi S, Okada Y.
Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics.
Molecular Biology Of The Cell
26(9), 1743-1751 (2015)
doi: 10.1091/mbc.E14-08-1287
Okada Y, Nakagawa S.
Super-resolution imaging of nuclear bodies by STED microscopy.
Methods in Molecular Biology
1262, 21-35 (2015)
doi: 10.1007/978-1-4939-2253-6_2
Uno S, Kamiya M, Yoshihara T, et al.
A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging.
Nature Chemistry
6(8), 681-689 (2014)
doi: 10.1038/nchem.2002
Yajima H, Ogura T, Nitta R, et al.
Conformational changes in tubulin in GMPCPP and GDP-taxol microtubules observed by cryoelectron microscopy.
Journal Of Cell Biology
198(3), 315-322 (2012)
doi: 10.1083/jcb.201201161
Hirokawa N, Tanaka Y, Okada Y.
Cilia, KIF3 molecular motor and nodal flow.
Current Opinion In Cell Biology
24(1), 31-39 (2012)
doi: 10.1016/j.ceb.2012.01.002
Nakata T, Niwa S, Okada Y, et al.
Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport.
Journal Of Cell Biology
194(2), 245-255 (2011)
doi: 10.1083/jcb.201104034
Hirokawa N, Nitta R, Okada Y.
The mechanisms of kinesin motor motility: lessons from the monomeric motor KIF1A.
Nature Reviews Molecular Cell Biology
10(12), 877-884 (2009)
doi: 10.1038/nrm2807
Nagao Y, Sakamoto M, Chinen T, et al.
Robust classification of cell cycle phase and biological feature extraction by image-based deep learning.