Team Director
Ichio Shimada
Ph.D.
Laboratory for Dynamic Structure of Biomolecules
[Affiliation has changed to RIKEN Center for Integrated Medical Sciences (IMS) as of April 2025]
LocationYokohama
E-mailichio.shimada@riken.jp
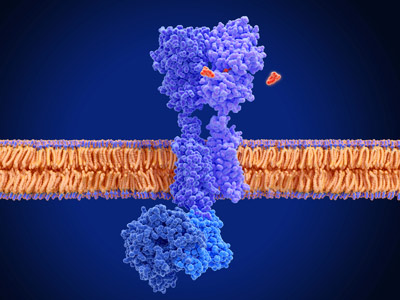
Membrane proteins play fundamental roles in many biological processes, and are recognized as principal target proteins for drug development. RNAs have become recognized asa novel remedy to solve the depletion of targets in drug development.Over the past decade, our structural understanding of biomolecules such as the membrane proteins and RNAshas dramatically progressed, owing to the growing numbers of their atomic resolution crystal and cryo-EM structures. However, these structures basically represent static snapshots and observed conformations may not be the same as those in in-situ environment. In this research team, by using NMR, which provides us information about dynamical structures of biomolecules in solution, we will investigate the relationships between the dynamical structures and the functions for biologically important biomolecules.
Research Theme
- Elucidation of functional mechanism of biomolecules from dynamic structures
- Elucidation of signal transduction mechanism of GPCRs from dynamic structures
- Development of methods for dynamic structures of membrane proteins in lipid bilayers
- Development of NMR methods applicable to high-molecular-weight biomolecules
Selected Publications
Toyama Y, Shimada I.
Quantitative analysis of the slow exchange process by 19F NMR in the presence of scalar and dipolar couplings: applications to the ribose 2'-19F probe in nucleic acids.
Journal of Biomolecular NMR
78(4), 215-235 (2024)
doi: 10.1007/s10858-024-00446-7
Kaneko S, Imai S, Uchikubo-Kamo T, et al.
Structural and dynamic insights into the activation of the μ-opioid receptor by an allosteric modulator.
Nature Communications
15, 3544 (2024)
doi: 10.1038/s41467-024-47792-6
Toyama Y, Shimada I.
NMR characterization of RNA binding property of the DEAD-box RNA helicase DDX3X and its implications for helicase activity.
Nature Communications
15, 3303 (2024)
doi: 10.1038/s41467-024-47659-w
Imai S, Suzuki H, Fujiyoshi Y, et al.
Dynamically regulated two-site interaction of viral RNA to capture host translation initiation factor.
Nature Communications
14, 4977 (2023)
doi: 10.1038/s41467-023-40582-6
Shiraishi Y, Shimada I.
NMR Characterization of the Papain-like Protease from SARS-CoV-2 Identifies the Conformational Heterogeneity in Its Inhibitor-Binding Site.
Journal of the American Chemical Society
145, 16669-16677 (2023)
doi: 10.1021/jacs.3c04115
Kaneko S, Imai S, Asao N, et al.
Activation mechanism of the μ-opioid receptor by an allosteric modulator.
Proceedings of the National Academy of Sciences
119(16), e2121918119 (2022)
doi: 10.1073/pnas.2121918119
Shiraishi Y, Kofuku Y, Ueda T, et al.
Biphasic activation of β-arrestin 1 upon interaction with a GPCR revealed by methyl-TROSY NMR
Nature Communications
12, 7158 (2021)
doi: 10.1038/s41467-021-27482-3
Iwahashi Y, Toyama Y, Imai S, et al.
Conformational equilibrium shift underlies altered K+ channel gating as revealed by NMR.
Nature Communications
11, 5168 (2020)
doi: 10.1038/s41467-020-19005-3
Mizukoshi Y, Takeuchi K, Tokunaga Y, et al.
Targeting the cryptic sites: NMR-based strategy to improve protein druggability by controlling the conformational equilibrium.
Science Advances
6(40), eabd0480 (2020)
doi: 10.1126/sciadv.abd0480
Imai S, Yokomizo T, Kofuku Y, et al.
Structural equilibrium underlying ligand -dependent activation of β2 -adreno receptor.
Nature Chemical Biology
16, 430-439 (2020)
doi: 10.1038/s41589-019-0457-5
Shimada I, Ueda T, Kofuku Y, et al.
GPCR drug discovery: integrating solution NMR data with crystal and cryo-EM structures.
Nature Reviews Drug Discovery
18(1), 59-82 (2019)
doi: 10.1038/nrd.2018.180