Team Director
Yu-Chiun Wang
Ph.D.
Laboratory for Epithelial Morphogenesis
LocationKobe / Developmental Biology Buildings
E-mailyu-chiun.wang@riken.jp
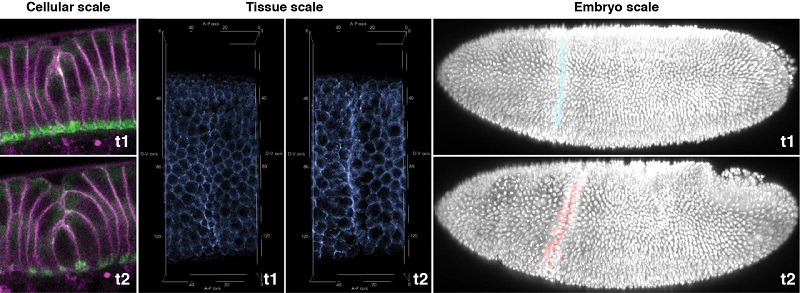
The central question in developmental biology is how cells, tissues and organs acquire their specific functions and shapes. A large body of work over the past several decades has yielded a broad understanding of how functional specialization is achieved through differential gene expression. In contrast, far less is known about how cell shapes and tissue structures are controlled and remodeled. Although a general theme has emerged whereby cytoskeletal elements control the cell shapes, while alteration of individual cell shapes collectively organizes the tissue architecture, the underlying molecular and mechanical mechanisms remain poorly understood. My lab aims at identifying novel mechanisms that orchestrate the formation of three-dimensional epithelial structures. Our long-term goal is to comprehensively understand the mechanistic principles of tissue morphogenesis in order to conceptualize the origin of morphological diversity both within an organism and among evolutionary lineages.
We are currently focusing on how modifications of epithelial cell polarity control cell shapes using gastrulating Drosophila embryos as the model system. Our recent work identified a novel microtubule based mechanical mechanism that is crucial for maintaining homogeneities of cell sizes and shapes prior to morphogenesis, and yet becomes coupled to the changing cell polarity, thus repurposed for cell shortening that induces folding of the epithelial tissue. The polarity-based, microtubule-dependent mechanism contrasts with the canonical myosin-dependent apical constriction. Our ongoing work promises to delineate a novel mechanical force balance/imbalance process that underlies non-myosin based epithelial folding.
We employ an integrated approach that combines genetic manipulation, quantitative live imaging and computational mechanical modeling. We are also in the process of developing optogenetic and cell-type specific RNA-seq methodologies that could be used to manipulate and identify factors and parameters that control cell shape change and tissue deformation. Furthermore, our lab is engaged in international, multidisciplinary collaborations with scientists that specialize in evolutionary biology, computational mechanics, and theoretical physics to seek to better understand epithelial morphogenesis from a variety of different angles.



Research Theme
- How does modification of cell polarity cause cell shape change and bending of an epithelial tissue?
- How does physical coupling between adherens junctions and actin control the extent of invagination?
- Developing strategies for quantitative 4D imaging and visualization of forces during morphogenesis
- The evolutionary possibilities of novel and temporary morphogenetic structures
Selected Publications
Eritano AS, Bromley CL, Bolea Albero A, et al.
Tissue-Scale Mechanical Coupling Reduces Morphogenetic Noise to Ensure Precision during Epithelial Folding.
Developmental cell
53(2), 212-228 (2020)
doi: 10.1016/j.devcel.2020.02.012
Takeda M, Sami M M, Wang YC.
A homeostatic apical microtubule network shortens cells for epithelial folding via a basal polarity shift.
Nature Cell Biology
20, 36-45. (2018)
doi: 10.1038/s41556-017-0001-3
Wen FL, Wang YC, Shibata T.
Epithelial folding driven by apical or basal-lateral modulation: geometric features, mechanical inference, and boundary effects.
Biophysical Journal
112, 2683-2695 (2017)
doi: 10.1016/j.bpj.2017.05.012
Wang YC, Khan Z, Wieschaus E F.
Distinct Rap1 activity states control the extent of epithelial invagination via alpha-catenin.
Developmental Cell
25, 299-309 (2013)
doi: 10.1016/j.devcel.2013.04.002
Wang YC, Khan Z, Kaschube M, Wieschaus E F.
Differential positioning of adherens junctions is associated with initiation of epithelial folding.
Nature
484, 390-3 (2012)
doi: 10.1038/nature10938
Wang YC, Ferguson E L.
Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning.
Nature
434, 229-34 (2005)
doi: 10.1038/nature03318
Members

Team DirectorYu-Chiun Wang
- yu-chiun.wang@riken.jp
Yu-Chiun Wang
Team Director
Chun Wai Kwan
Research Scientist
Sameer Thukral
Research Scientist
Bipasha Dey
Postdoctoral Researcher
Anne Rosfelter
Postdoctoral Researcher
Michiko Takeda
Technical Staff I
Nada Dougui
Research Associate
Anshuman Mishra
Research Associate
Yuko Fujiyama
Assistant