Team Leader
Yuko Mimori-Kiyosue
Ph.D.
Laboratory for Molecular and Cellular Dynamics
[Closed Mar. 2023]
E-mailyuko.kiyosue[at]riken.jp
Please replace [at] with @.
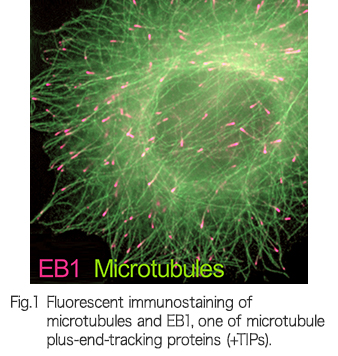
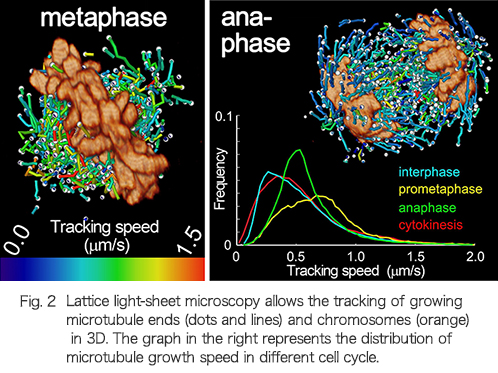
It is the working capacity of individual cells in our body that ensures we remain healthy or causes disease. The activities of cells are expressed through signal transduction, infrastructure construction and their dynamic regulation in a spatiotemporal manner. We are exploring the molecular and cellular mechanisms underlying normal development and diseases by employing a 3D high-resolution live-imaging technology, “lattice light-sheet microscopy”, which provides cellular information with unprecedented accuracy and precision. Currently, progress to establish a new style of cell biology using a large quantity of multidimensional information from lattice light-sheet microscopy is ongoing.
Research Theme
- Functional study of intracellular machinery driving life
- Research on molecular mechanisms that maintain cellular integrity and homeostasis
- Development of a quantitative cell phenotyping method using lattice light-sheet microscopy
Selected Publications
Yamashita N, Morita M, Legant WR, et al.
Three-dimensional tracking of plus-tips by lattice light-sheet microscopy permits the quantification of microtubule growth trajectories within the mitotic apparatus.
Journal of Biomedical Optics
20(10), 101206 (2015)
doi: 10.1117/1.JBO.20.10.101206
Chen BC, Legant WR, Wang K, et al.
Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution.
Science
346(6208), 1257998 (2014)
doi: 10.1126/science.1257998
Shimozawa T, Yamagata K, Kondo T, et al.
Improving spinning disk confocal microscopy by preventing pinhole cross-talk for intravital imaging.
Proceedings of the National Academy of Sciences of the United States of America
110(9), 3399-3404 (2013)
doi: 10.1073/pnas.1216696110
Nakamura S, Grigoriev I, Nogi T, et al.
Dissecting the nanoscale distributions and functions of microtubule-end-binding proteins EB1 and ch-TOG in interphase HeLa cells.
PLOS ONE
7(12), e51442 (2012)
doi: 10.1371/journal.pone.0051442
Mimori-Kiyosue Y.
Shaping microtubules into diverse patterns: molecular connections for setting up both ends.
Cytoskeleton (Hoboken)
68(11), 603-618 (2011)
doi: 10.1002/cm.20540
Hotta A, Kawakatsu T, Nakatani T, et al.
Laminin-based cell adhesion anchors microtubule plus ends to the epithelial cell basal cortex through LL5α/β.
The Journal of Cell Biology
189(5), 901-917 (2010)
doi: 10.1083/jcb.200910095
Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, et al.
CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex.
The Journal of Cell Biology
168(1), 141-153 (2005)
doi: 10.1083/jcb.200405094
Mimori-Kiyosue Y, Tsukita S.
Search-and-capture of microtubules through plus-end-binding proteins (+TIPs).
The Journal of Biochemistry
134(3), 321-326 (2003)
doi: 10.1093/jb/mvg148
Mimori-Kiyosue Y, Shiina N, Tsukita S.
The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules.
Current Biology
10(14), 865-868 (2000)
doi: 10.1016/S0960-9822(00)00600-X
Mimori-Kiyosue Y, Shiina N, Tsukita S.
Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells.
The Journal of Cell Biology
148(3), 505-518 (2000)
doi: 10.1083/jcb.148.3.505
Members
Yoshihiro Kawasaki
Senior Research Scientist
Akiko Hayashi
Research Part-time Worker I