Marcksl1 keeps blood vessels in shape
Mar. 26, 2021
The development, function and survival of tissues depend on blood vessels that deliver oxygen and nutrients to all corners of our body. Therefore, the failure to form or maintain a well-ordered network of tubular blood vessels is detrimental to tissue physiology and consequently, health. Endothelial cells form the innermost lining of blood vessels and are subjected to the mechanical stresses of pressurized blood flow (hemodynamic forces), which in turn influence endothelial cell behavior. For example, blood pressure induces spherical membrane protrusions, called blebs, in the apical membranes during lumen expansion of newly forming blood vessels*1. These blebs occur in regions of the apical membranes with low actomyosin activity, and the timely retraction of these blebs is also essential for normal progression of lumen expansion. However much of the molecular mechanisms involved in how endothelial cells regulate lumen expansion remained unclear.
In a recent study led by RIKEN BDR’s Laboratory for Vascular Morphogenesis (Li-Kun Phng, Team Leader), research scientist Igor Kondrychyn and colleagues demonstrate that endothelial cells develop a protective mechanism to resist the deforming forces of blood flow to control cell and vessel morphology during vessel development. Using zebrafish, they identify an actin-binding protein, Myristoylated alanine-rich C-kinase (Marcksl1), as the molecule involved in modulating this endothelial cell resistance by regulating cortical actin organization and cortical tension. Their work was published in the online journal, Nature Communications.
The team first noticed differences in actomyosin activity in the apical cortex of endothelial cells at distinct stages of lumen formation (or expansion) in zebrafish embryos using live imaging—low actomyosin activity was observed in the apical cortex in early stages of lumen expansion and higher levels were detected at later stages when vessels become perfused. Looking for possible proteins regulating these membrane dynamics during lumen expansion, they became interested in Marcksl1, an actin-bundling protein reported to be expressed in endothelial cells of zebrafish during development. When Marcksl1 localization in endothelial cells was examined during lumen formation, they saw that while initially diffused in the cytoplasm, Marcksl1 became enriched in the apical membrane as lumen expansion progressed, suggesting that it may play a role there.
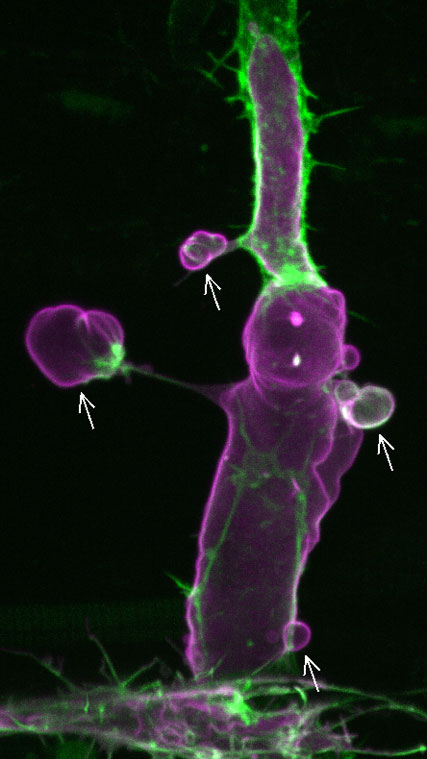
Figure 1. (Movie) A fluorescent image of an intersegmental vessel of a 2-day post-fertilization zebrafish embryo showing endothelial cells overexpressing Marcksl1 (magenta). Arrows point to abnormal basal membrane blebs. Green, F-actin.
The team investigated the function of Marcksl1 and found that overexpression or high levels of the protein in endothelial cells leads to basal membrane blebbing in perfused vessels (Figure 1), a behavior that is not observed in normal vessels. Marckl1-induced membrane blebs show dynamic changes in shape and size, suggesting that there is active reorganization of the actomyosin network. Indeed, they found that there is little actin in the membrane cortex when blebs initially form. As the bleb expands, actin polymerization increases from the base towards the periphery of the bleb. When the entire bleb is covered by actin, the bleb retracts. Likewise, myosin II, initially seen accumulated at the base of the bleb, gradually expanded around the bleb before bleb retraction (Figure 2). As the formation of basal membrane blebs was also observed when the endothelial cell cortex was ablated using a laser, this suggested that high levels of Marcksl1 weakens the cell cortex so that it is unable to withstand hemodynamic forces. This was further supported by experiments in which the team altered blood flow (i.e. pressure and shear stress) in embryos with Marcksl1-overexpressed endothelial cells by treating them with high or low doses of an anesthetic drug, tricaine—reduction of blood flow resulted in a gradual decline of the number of blebs being formed, and the resumption of blood flow and thereby hemodynamic forces caused more apical membrane blebbing.
Figure 2. (Movie) Myosin light chain 9b (green) dynamics in a Marcksl1-induced bleb. Magenta, Marcksl1-overexpressing endothelial cell.
Noting that the overexpression of Marcksl1 also appeared to produce endothelial cells with bulbous shape, the authors carried out gain-of-function and loss-of-function experiments to clarify this relationship. They demonstrated that elevating Marcksl1 expression in endothelial cells increased endothelial cell size as well as blood vessel diameter while depleting Marcksl1 in endothelial cells led to slightly smaller endothelial cell size and a significant reduction in vessel diameter.
Seeking to understand how Marcksl1 regulates membrane behavior and cell size, the team examined the actin organization in the endothelial cell cortex. High-resolution imaging revealed that Marcksl1 promotes the formation of linear actin bundles but decreases actin density at the cell cortex so that these regions become highly deformable when exposed to blood flow, and are therefore unable to maintain membrane and cell shape.
"Proper lumen formation and maintenance is critical for blood vessels to perform their functions, and our study reveals a novel mechanism by which the endothelial cells lining the inside of vessels generate actin-dependent resistive forces to oppose hemodynamic forces to regulate vessel shape and size," says Phng. "And this protective mechanism has clinical implications in vascular diseases such as aneurysms that display altered vessel morphologies as a result of weakening of the blood vessel wall."
By Hazuki S. Hiraga
*1 Gebala V, Collins R, Guedens I, Phng L-K & Gerhardt H: Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nature Cell Biology 18, 443–450 (2016)
Publication Information
Kondrychyn I, Kelly DJ, Carretero NT, et al.
Marcksl1 modulates endothelial cell mechanoresponse to haemodynamic forces to control blood vessel shape and size.
Nature Communications 11, 5476 (2020) doi: 10.1038/s41467-020-19308-5



